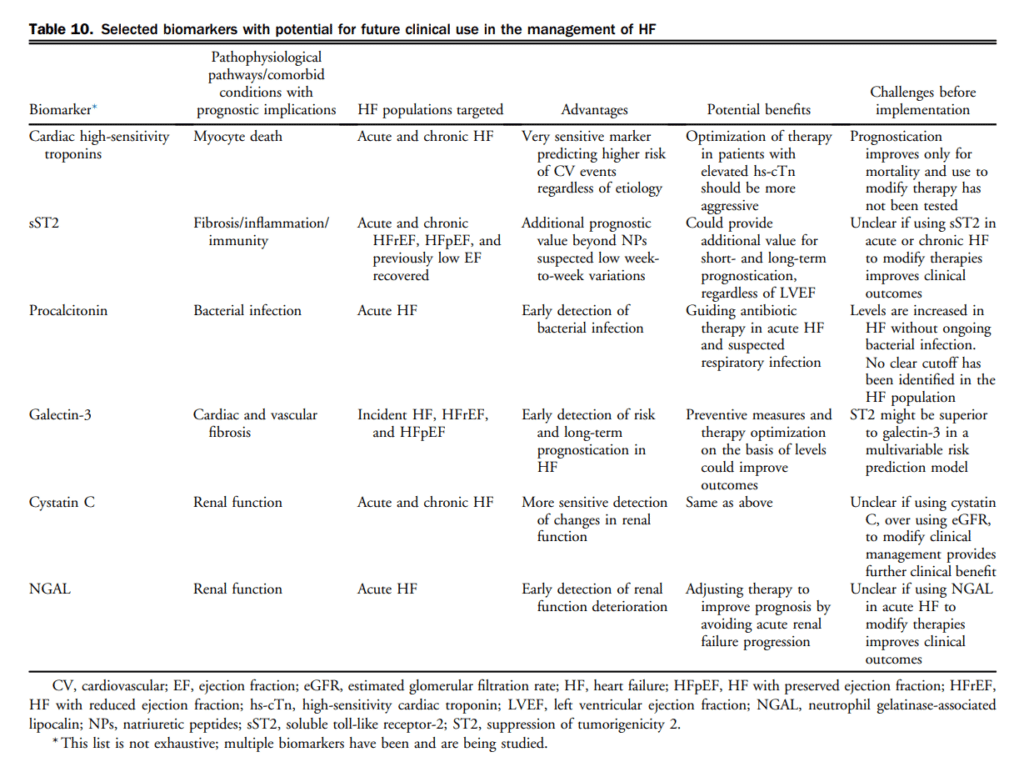
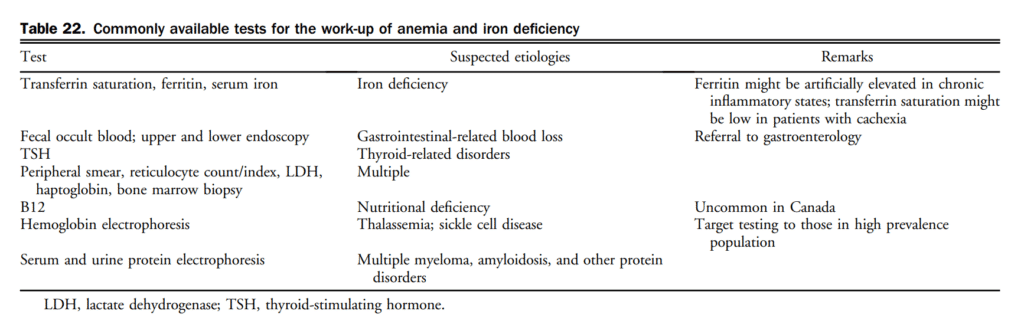
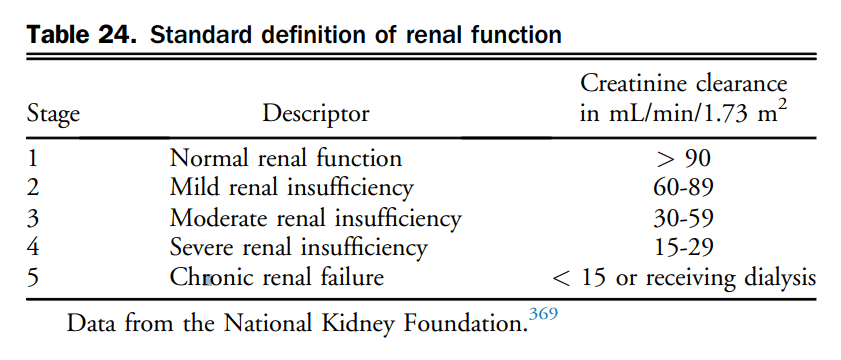
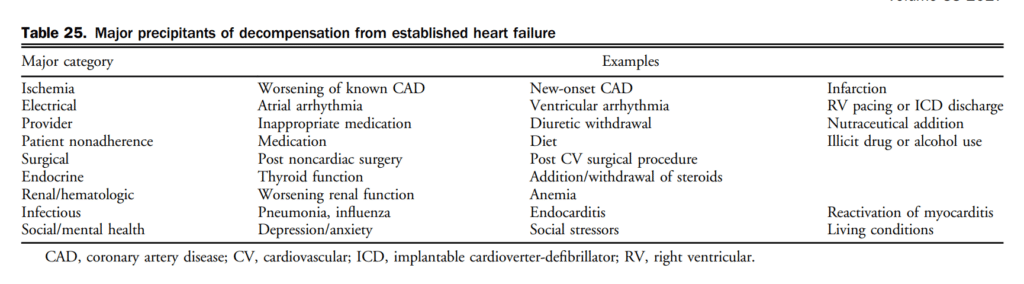
7. Treatment
7.1 Chronic HF
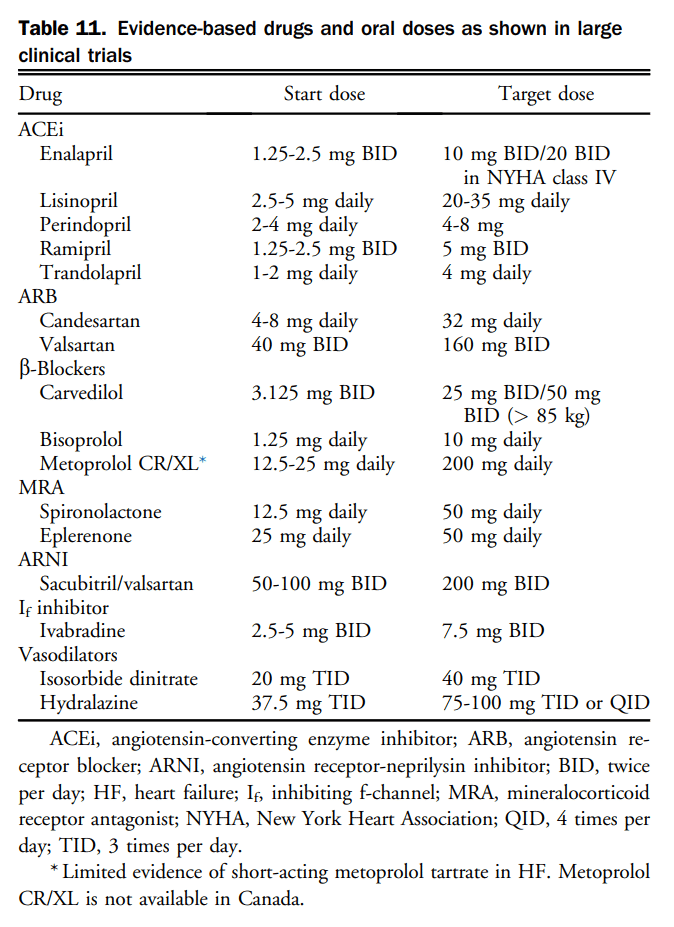
Pharmacotherapy has been shown to change the natural history of HFrEF. HFpEF however, has been identified as major public health issue and to date, the etiology, diagnosis, characterization, and treatment has remained challenging. Goals of HF therapy include improving survival and reducing morbidity such as hospitalizations and symptoms, while improving functional capacity and quality of life. Figure 4 shows a therapeutic approach to patients with HFrEF that is considered optimal medical therapy and defined as GDMT throughout this section. The evidence-based medications and doses of GDMT are shown in Table 11.
7.1.1 HFrEF pharmacological treatment
Contemporary treatment for most patients with HFrEF encompasses triple therapy, which includes the combination of: (1) an ACEi (or ARB if ACEi-intolerant); (2) a β-blocker; and (3) an MRA. Working on various pathways of the neurohormonal system, the combination of these agents has been shown to improve survival in patients with HFrEF. There are many landmark trials and meta-analyses that support the use of ACEis[93]–[99] and β-blockers[100]–[104] in all patients across the spectrum of HFrEF. ARBs have been shown to be superior to placebo in those intolerant to ACEis and are considered a good second-line agent.[105]–[108] Likewise, there are 2 key clinical trials and 1 meta-analysis[109]–[111] that support the additional use of an MRA with this combination with an improvement in survival across the spectrum of symptomatic patients with HFrEF. Most recently, the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) expanded the use of aldosterone receptor antagonists in HFrEF patients with mild symptoms.[110] In EMPHASIS-HF the effects of eplerenone on clinical outcomes were examined in patients 55 years of age or older, with NYHA II symptoms, LVEF < 30% (if > 30%-35%, a QRS duration of > 130 ms), treated with an ACEi and/or ARB, and β-blockers. A total of 2737 patients with a median follow-up of 21 months were enrolled. There was a 37% reduction in the primary composite outcome of death from cardiovascular causes or first hospitalization for HF with eplerenone.
7.1.1.1 Pharmacologic therapy
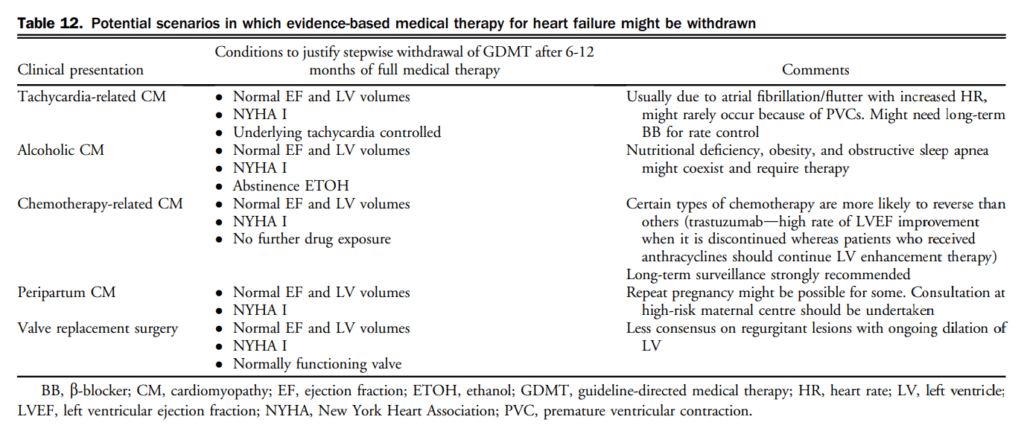
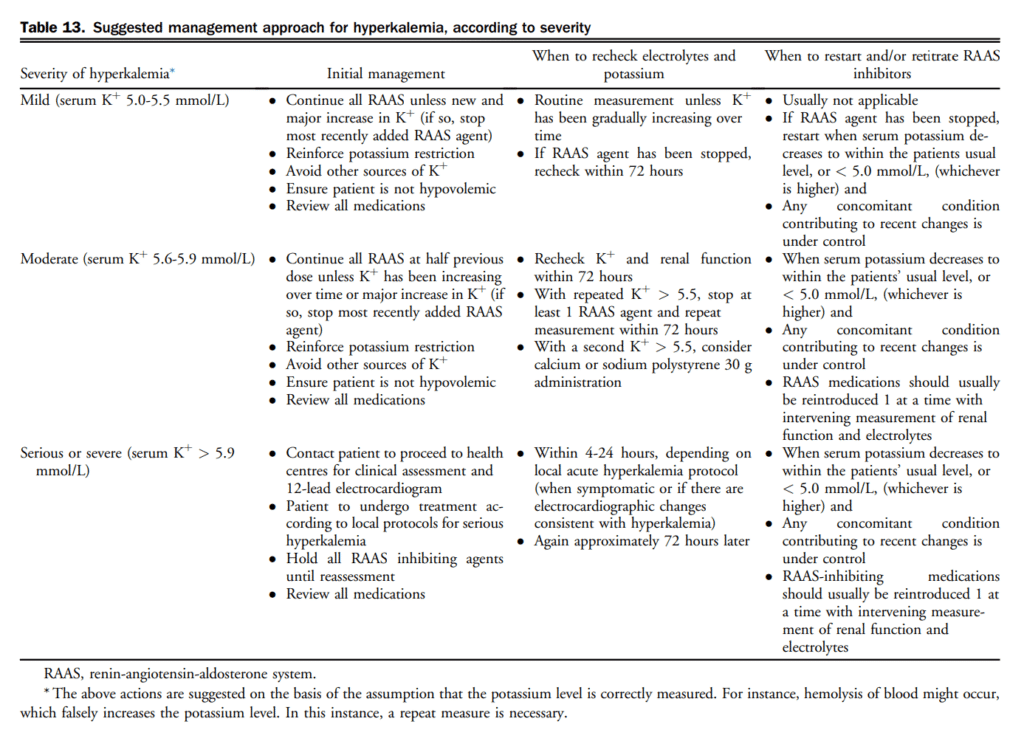
In addition to initiating, titrating, and monitoring pharmacologic therapy, there are circumstances in which some therapies may be withdrawn (Table 12). There are additionally some common effects of GDMT requiring active surveillance and management. A suggested approach to hyperkalemia is presented in Table 13.
Recommendation
26. We recommend that most patients with HFrEF be treated with triple therapy including an ACEi (or an ARB in those who are ACEi-intolerant), a β-blocker and an MRA unless specific contraindications exist (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
Preference is given to the use of pharmacotherapy in most patients with HFrEF across the spectrum of symptoms. There are limited clinical trial data to inform decision-making surrounding the use of MRAs as part of GDMT in those without symptoms of HF or high-risk features.
27. We recommend preferentially using the specific drugs at target doses that have been proven to be beneficial in clinical trials as optimal medical therapy. If these doses cannot be achieved, the maximally tolerated dose is acceptable (Table 11) (Strong Recommendation; High-Quality Evidence).
Practical Tip (general)
If a drug with proven mortality or morbidity benefits does not appear to be tolerated by the patient (eg, low BP, low heart rate, or renal dysfunction), other concomitant drugs, including diuretics, with less proven benefit should be carefully re-evaluated to determine whether their dose can be reduced or the drug discontinued.
Practical Tip (general)
HFrEF GDMT should be continued at the usual dose during acute intercurrent illness (eg, pneumonia, exacerbation of chronic obstructive pulmonary disease, other systemic infection, etc), unless they are not tolerated (eg, if significant reactive airway disease is present). GDMT should be restarted before discharge if temporarily withheld.
Practical Tip (general)
In a life-threatening complication, GDMT may be discontinued abruptly, but generally, if there is concern about their use, the dose should be decreased by one-half, and the patient should be reassessed. If the dose is reduced, it should be uptitrated to the previous tolerated dose as soon as safely possible.
Practical Tip (general)
If symptomatic hypotension persists with GDMT, consider separating the administration of the dose from the timing of other medications that could also lower BP.
Practical Tip (ACEi/ARB)
ACEi intolerance describes a patient who is unable to tolerate ACEi therapy secondary to a bothersome cough (most commonly, 10%-20%) or those who experience angioedema with ACEi therapy (uncommon; < 1%). ARB therapy is a reasonable alternative in both of these cases, however, caution should be used in patients who develop angioedema while receiving ACEi therapy because there have been case reports of patients who subsequently develop angioedema with ARB therapy. There is no significant difference in rates of hypotension, hyperkalemia, or renal dysfunction between these agents to warrant a substitution between agents.
Practical Tip (ACEi/ARB)
An increase in serum creatinine or eGFR of up to 30% is not unexpected when an ACEi or ARB is introduced; if the increase stabilizes at ≤ 30%, there is no immediate need to decrease the drug dose but closer long-term monitoring might be required.
Practical Tip (ACEi/ARB)
BP might lower when an ACEi or ARB is introduced, especially if introduced at a high dose or in combination with diuretic therapy. Check BP with the patient supine and erect to detect whether hypotension is present, requiring slower uptitration.
Practical Tip (ACEi/ARB)
Cough occurs in 10%-20% of patients receiving ACEis and does not require discontinuation of the agent unless it is bothersome to the patient.
Practical Tip (β-blockers)
Objective improvement in cardiac function might not be apparent for 6-12 months after b-blocker initiation.
Practical Tip (β-blockers)
Patients in NYHA class I or II can be safely initiated and titrated with a b-blocker by nonspecialist physicians.
Practical Tip (β-blockers)
Patients in NYHA class III or IV should have their b-blocker therapy initiated by a specialist experienced in HF management and titrated in the setting of close follow-up, such as can be provided in a specialized clinic, if available.
Practical Tip (β-blockers)
The starting dose of b-blockers should be low and increased slowly (eg, double the dose every 2-4 weeks). Transient fluid retention might occur with initiation or uptitration of b-blockers and might require assessment of diuretic dosage (eg, might consider deferring dosage reduction).
Practical Tip (β-blockers)
If concomitant reactive airways disease is present, consider using more selective b-1 blockade (eg, bisoprolol).
Practical Tip (β-blockers)
If atrioventricular (AV) block is present, consider decreasing other AV-blocking drugs, such as digoxin or amiodarone (when appropriate). The type and severity of AV block and the patient’s history of arrhythmias will help guide the most appropriate treatment modifications.
Practical Tip (MRA)
MRAs can increase serum potassium, especially during an acute dehydrating illness in which renal dysfunction can worsen, and close monitoring of serum creatinine and potassium is required. High-risk groups include those with diabetes, pre-existing renal dysfunction, and older age.
7.1.1.2 ACEi/ARB
There are extensive data on the use of ACEi and β-blocker treatment for patients with HFrEF to reduce morbidity and mortality and improve quality of life.[112],[113] A notable deletion from these guidelines is the recommendation to consider combination ACEi and ARB therapy, previously recommended. The combination of an ACEi with an ARB is no longer recommended. Although some evidence exists to support a reduction in clinically relevant outcomes with the combination, there is also substantial evidence that was published after the previous recommendation, outlining harm in terms of adverse effects (eg, hypotension, hyperkalemia, and renal dysfunction).[108],[114],[115] More contemporary treatments with MRAs and ARNIs have a stronger evidence base across the spectrum of outcomes (eg, morbidity and mortality) and therefore further limit the role of combination ACEi and ARB therapy.
Recommendation
28. We recommend an ACEi, or ARB in those with ACEi intolerance, in patients with acute MI with HF or an EF < 40% post-MI to be used as soon as safely possible post-MI and be continued indefinitely (Strong Recommendation; High-Quality Evidence)
7.1.1.3 β-Adrenergic receptor blocker (β-blocker)
β-Blockers are part of the first-line therapy in the treatment of HFrEF, because they have been proven to improve survival and decrease hospitalizations in this population of patients, in a number of large clinical trials.[101],[103],[116]-[121]
Recommendation
29. We recommend NYHA class IV patients be stabilized before initiation of a β-blocker (Strong Recommendation; High-Quality Evidence).
30. We recommend that β-blockers be initiated as soon as possible after diagnosis of HF, including during the index hospitalization, provided that the patient is hemodynamically stable. Clinicians should not wait until hospital discharge to start a β-blocker in stabilized patients (Strong Recommendation; High-Quality Evidence).
31. We recommend that β-blockers be initiated in all patients with an LVEF < 40% with previous MI (Strong Recommendation; Moderate-Quality Evidence).
7.1.1.4 MRAs
A single RCT supports the use of eplerenone (target 50 mg daily) compared with placebo post-MI.[122] The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) trial enrolled 6642 patients who had an MI 3-14 days previously with an LVEF < 40% and symptoms of HF or an LVEF < 30% and diabetes without symptoms of HF. The primary outcome included all-cause mortality and cardiovascular mortality or hospitalization for cardiovascular events. After a median follow-up of 16 months, there was a 15% relative decrease in mortality and 13% relative decrease in cardiovascular mortality or hospitalization for cardiovascular events in the eplerenone group. There was more hyperkalemia in the eplerenone group.
Recommendation
32. We recommend an MRA for patients with acute MI with EF < 40% and HF or with acute MI and an EF < 30% alone in the presence of diabetes (Strong Recommendation; High-Quality Evidence).
7.1.1.5 ARNI
In those who remain symptomatic despite triple therapy, consideration should be made to change an ACEi/ARB to an ARNI. Neprilysin, a neutral endopeptidase, degrades several endogenous vasoactive peptides, including NPs, bradykinin, and adrenomedullin. Inhibition of neprilysin increases the levels of these substances, countering the neurohormonal overactivation that contributes to vasoconstriction, sodium retention, and maladaptive remodelling.[123],[124] In the Prospective Comparison of ARNi With ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial, the ARNI sacubitril/valsartan was compared with enalapril in patients with HFrEF.[125] A total of 8442 patients were randomized to sacubitril/valsartan 200 mg twice daily or enalapril 10 mg twice daily after a 6-8 week runin phase. Patients were included if they were NYHA class II-IV (70% class II), LVEF ≤ 40% (amended to ≤ 35%), had a BNP ≥ 150 pg/mL (or NT-proBNP ≥ 600 pg/mL), or hospitalization for HF in the past year and BNP ≥ 100 pg/mL (or NT-proBNP ≥ 400 pg/mL). The primary outcome was a composite of death from cardiovascular causes or hospitalization for HF. The trial was stopped early, according to prespecified rules, after a median follow-up of 27 months. The primary outcome occurred in 914 patients (21.8%) in the sacubitril/valsartan group and 1117 patients (26.5%) in the enalapril group, a 20% relative reduction. There was also a decrease in all-cause mortality, cardiovascular mortality, HF hospitalization, and symptoms of HF. The sacubitril/valsartan group had a higher proportion of patients with hypotension but a smaller risk of renal impairment, hyperkalemia, and cough than the enalapril group. The type of patients and magnitude of effect were similar to other landmark trials in HFrEF including ACEis, β-blockers, and MRAs. This trial also closely reflects contemporary practice with high utilization of ACEis, β-blockers, and MRAs (100%, 92%, and 55%, respectively) at baseline and had an active gold standard comparator.
Recommendation
33. We recommend that an ARNI be used in place of an ACEi or ARB, in patients with HFrEF, who remain symptomatic despite treatment with appropriate doses of GDMT to decrease cardiovascular death, HF hospitalizations, and symptoms (Strong Recommendation; High-Quality Evidence).
Values and Preferences
This recommendation places high value on medications proven in large trials to reduce mortality, HF re-hospitalization, and symptoms. It also considers the health economic implications of new medications.
Practical Tip
Drug tolerability, side effects, and laboratory monitoring with use of ARNIs is similar to that of ACEi or ARB noted previously.
The PARADIGM-HF trial excluded patients with a serum potassium > 5.2 mmol/L, an eGFR < 30 mL/min, and symptomatic hypotension with a systolic BP of < 100 mm Hg.
When switching between an ARNI and an ACEi, a washout period of at least 36 hours is required to decrease the risk of angioedema. No washout period is required for conversion between ARNIs and ARBs.
ARNIs should not be used in anyone with a history of angioedema.
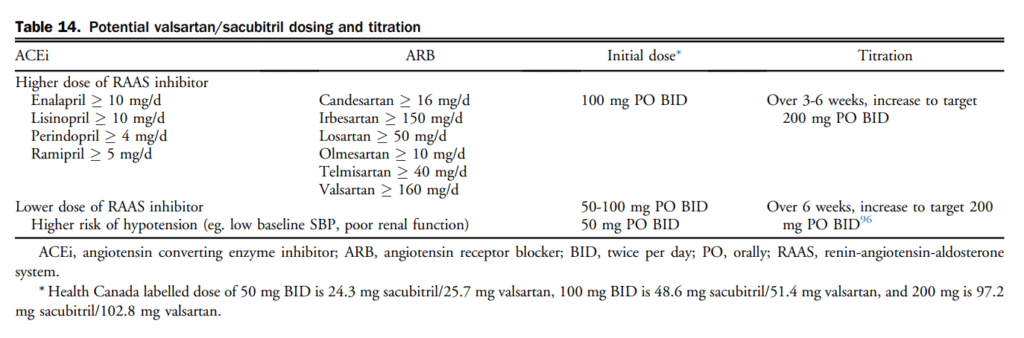
Currently, there is only 1 ARNI, sacubitrilvalsartan, available on the Canadian market. Initial dosing and rate of titration is dependent on pre-existing treatment and comorbidities and should be individualized (Table 14). When selecting a dose or titration schedule consideration should be given to the likelihood of tolerability and ultimately successful titration to doses shown to improve important HF outcomes.
7.1.1.6 Ivabradine
Resting heart rate independently predicts CVD events, including HF hospitalization.[126],[127] Systematic reviews have shown that a major contributor to the benefits of b-blocker therapy might be their rate-lowering effect.[128]–[130] Despite their benefits, β-blockers are generally underused and underdosed.[129]–[131] Ivabradine is approved for the treatment of HF by Health Canada. The latter drug selectively inhibits the depolarizing If current in the sinus node. It thus requires sinus rhythm to provide its pharmacological effect. In contrast to β-blockers, ivabradine does so without lowering BP or myocardial contractility.[132],[133]
The first trial to assess ivabradine in CAD was the Morbidity-Mortality Evaluation of the If Inhibitor Ivabradine in Patients With Coronary Disease and Left-Ventricular Dysfunction (BEAUTIFUL) trial.[134] In this trial the effect of ivabradine 7.5 mg twice daily was evaluated in patients with CAD and LVEF < 40% in sinus rhythm with a heart rate > 60 bpm in > 10,000 patients. Although ivabradine did not reduce the primary composite end point of cardiovascular death, hospitalization for MI, or new-onset or worsening HF, it did reduce the incidence of the secondary end point of fatal and nonfatal MI in patients with a baseline heart rate ≥ 70 bpm.
The Systolic Heart Failure Treatment With the If Inhibitor Ivabradine Trial (SHIFT) trial was the key trial to address the use of ivabradine in symptomatic HF.[135] Inclusion criteria were NYHA class II-IV, sinus rhythm, resting heart rate ≥ 70 bpm, LVEF ≤ 35%, and HF admission within 12 months. Patients were randomized to a target dose of ivabradine 7.5 mg twice daily vs placebo. The primary end point was a composite of cardiovascular death or HF admission. Ninety percent of patients were receiving a β-blocker, and 56% were receiving 50% of target doses. Heart rate was 8 bpm lower in the ivabradine group at the end of the study. There was an 18% decrease in the primary outcome, which was largely driven by hospital admission for worsening HF (RRR, 26%). Treatment effect was consistent across prespecified subgroups, although the difference between treatment groups did not reach statistical significance in the subgroup with a baseline heart rate lower than the median of 77 bpm. Additionally, in those receiving > 50% of the target dose of a β-blocker, the overall trial results were similar. Ivabradine did not reduce all-cause or cardiovascular mortality. There were more withdrawals (21% vs 19%) and bradycardia in the ivabradine group (10% vs 2%). Only 1% of patients withdrew from the study as a consequence of bradycardia. Visual symptoms specific to ivabradine occurred rarely (3% vs 1% with placebo; P < 0.0001 and led to withdrawal in 1% of cases).
Recommendation
34. We recommend that ivabradine be considered in patients with HFrEF, who remain symptomatic despite treatment with appropriate doses of GDMT, with a resting heart rate > 70 beats per minute (bpm), in sinus rhythm, and a previous HF hospitalization within 12 months, for the prevention of cardiovascular death and HF hospitalization (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
High value is placed on the improvement of cardiovascular death and HF hospitalizations as adjunctive therapy to standard HF medication treatments in a selected HF population. The health economic implications are unknown. Differing criteria for heart rate eligibility have been approved by various regulatory authorities ranging from 70 to 77 bpm with the trial entry criteria of 70 bpm.
Practical Tip
Every effort should be made to achieve target or maximally tolerated doses of b-blockers before initiation of ivabradine.
Ivabradine has no effect on BP or myocardial contractility.
7.1.1.7 Hydralazine and isosorbide dinitrate
Three RCTs inform the use of H-ISDN in HFrEF. The Vasodilator in Heart Failure Trial (V-HeFT) trial, the first RCT, compared the effect of H-ISDN, prazosin and placebo in HFrEF on mortality (n = 642).[136] After a mean follow-up of 2.3 years, there was no difference in mortality for the entire follow-up period (primary outcome), but showed a 66% relative improvement in survival in the H-ISDN group at 2 years. This trial predated the era of ACEis and β-blockers. The second trial to evaluate H-ISDN (300 mg and 160 mg) compared with enalapril (20 mg daily) in HFrEF on the outcome of mortality (n = 804).[137] There was a reduction in mortality in the enalapril arm after a mean of 2.5 years (32.8% vs 38.2%; P = 0.016) and no difference in hospitalizations. Neither of these trials provide an insight into the role of H-ISDN in the face of contemporary therapy. The third trial was the African-American Heart Failure Trial (A-HeFT) trial, in which H-ISDN was investigated in additional to optimal therapy (ACEi/ARB, β-blocker, MRA) in self-identified black patients with NYHA class III/IV HFrEF.[138] Black patients were specifically evaluated in this trial because it had been noted that this population has a less active renin-angiotensin system and seemed to respond better to H-ISDN. In this trial H-ISDN (225 mg or 120 mg) was evaluated vs placebo (in addition to standard therapy) on the outcome of all-cause mortality, first hospitalization for HF, and quality of life. A total of 1050 black patients were enrolled and followed for a mean of 10 months. The study was terminated early secondary to higher mortality in the placebo group. The primary outcome was a weighted score, but individual components of the outcome showed a difference favouring H-ISDN for all-cause mortality, first hospitalization for HF, and change in quality of life score. It is unclear if these results can be extrapolated to other groups.
Recommendation
35. We recommend the combination of hydralazine and isosorbide dinitrate (H-ISDN) be considered in addition to standard GDMT at appropriate doses for black patients with HFrEF and advanced symptoms (Strong Recommendation; Moderate-Quality Evidence).
36. We recommend that H-ISDN be considered in patients with HFrEF who are unable to tolerate an ACEi, ARB, or ARNI because of hyperkalemia or renal dysfunction (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
There is limited high-quality clinical trial evidence in the modern era from which to base an H-ISDN recommendation without considering the tolerability and adverse effects. Adverse effects related to H-ISDN are frequent, limit uptitration, and result in discontinuation in a significant proportion of patients. Every effort should be made to use ACEi/ARB/ARNI therapy including a low dose and/or rechallenge therapy before changing to H-ISDN.
Practical Tip
Renal dysfunction warranting a trial of H-ISDN includes those who have a significant change in creatinine from baseline with ACEi/ARB/ARNI therapy that persists despite modification of dose, rechallenge, and/or removal of other potentially nephrotoxic agents. It may also be considered in those with a serum creatinine (Scr) > 220 mmol/L who experience significant worsening in renal function with the use of ACEi/ARB/ARNI therapy, or in a trial of these agents (eg, potential worsened renal function requiring renal replacement therapy) is thought to outweigh benefits.
Hyperkalemia warranting a trial of H-ISDN includes those with persistent hyperkalemia (K > 5.5 mmol/L) despite dietary intervention, dosage reduction of ACEi/ARB/ARNI, and removal of other agents known to increase potassium levels.
Nitrates alone might be useful to relieve orthopnea, paroxysmal nocturnal dyspnea, exercise-induced dyspnea, or angina in patients when used as tablet, spray, or transdermal patch, but continuous (ie, around the clock) use should generally be avoided because most patients will develop tolerance.

7.1.1.8 Digoxin
The effect of digoxin on mortality and morbidity in patient with heart failure (Digitalis Investigation Group [DIGtrial])[139] enrolled 6800 patients with HF and a LVEF ≤ 45% and were randomized to digoxin (median dose 0.25 mg/d) or placebo. The primary outcome was mortality over a mean follow-up of 37 months. Fifty-four percent were NYHA class II and 94% of patients were receiving an ACEi. There was no difference in all-cause mortality. There was a decrease in HFrelated deaths but an increase in “other cardiac deaths,” which has led to speculation that it might be due to arrhythmic death and led to an overall neutral effect on mortality. There were fewer patients hospitalized for HF in the digoxin group. Suspected digoxin toxicity was higher in the digoxin group (11.9% vs 7.9%). A systematic review included 13 studies (n = 7896, 88% of participants from the DIG-trial) showed similar results.[140] None of these studies provide much insight into the relative benefit or harm of digoxin in light of contemporary therapy with β-blockers and MRAs, however, many landmark trials of these agents had a substantial background therapy of digoxin with no apparent change in the overall results if a patient was or was not receiving digoxin.
Recommendation
37. We suggest digoxin be considered in patients with HFrEF in sinus rhythm who continue to have moderate to severe symptoms, despite appropriate doses of GDMT to relieve symptoms and reduce hospitalizations (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendations place a high value on the understanding that the use of cardiac glycosides in HFrEF remains controversial in light of contemporary therapy, and digoxin had no effect on mortality, cardiovascular hospitalizations, exercise, or the primary end point in DIG-trial. Digoxin can cause atrial and ventricular arrhythmias particularly in the presence of hypokalemia or in the presence of worsening of renal function (with increased digoxin levels).
Practical Tip
In patients receiving digoxin, serum potassium and creatinine should be measured with increases in digoxin or diuretic dose, the addition or discontinuation of an interacting drug, or during a dehydrating illness, to reduce the risk of digoxin toxicity. Patients with reduced or fluctuating renal function, elderly patients, those with low body weight, and women are at increased risk of digoxin toxicity and might require more frequent monitoring including digoxin levels.
Routine digoxin levels are not required other than to assess for digoxin toxicity. Digoxin levels should not be used to guide chronic therapy. Titration to digoxin levels has not been tested in clinical trials.
7.1.1.9 Omega-3 polyunsaturated fatty acid
The Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza cardiaca-Heart Failure (GISSI-HF) study was an RCT designed to assess the effects of omega-3 polyunsaturated fatty acids (n-3 PUFAs) in HF.[141] More than 4600 patients with NYHA class II to IV HF, irrespective of etiology or EF, were randomly assigned to a fish-based n-3 PUFA (daily 850 mg to 882 mg eicosapentaenoic acid and docosahexaenoic acid as ethyl esters in the average ratio of 1:1.2) or placebo. The primary end points were time to death, and time to death or admission to hospital for cardiovascular reasons. After a median 3.9-year follow-up, there was a decrease in both primary outcomes favouring n-3 PUFA (9% relative reduction in all-cause death and an 8% relative reduction in death or admission to hospital). The therapy was well tolerated with primarily gastrointestinal side effects, and fewer than 10% of patients required study drug withdrawal. Current sources of n-3 PUFA in Canada are food supplements, therefore, are not subject to the regulatory review (including predefined tolerances for drug content) that is required for any drug approval. As such, it is difficult to be certain of the amount of n-3 PUFA present in any given commercial preparation. Indeed, evidence suggests a large degree of variability between different available forms of n-3 PUFA.[142] Patients and caregivers who wish to use n-3 PUFA are therefore referred to a local medical practitioner, pharmacy, or other reputable source of information to determine their best source of n-3 PUFA. Reports of excessive bleeding have been associated with doses < 3 g/d, but this remains controversial.[143],[144]
Recommendation
38. We suggest n-3 PUFA therapy at a dose of 1 g/d be considered for reduction in morbidity and cardiovascular mortality in patients with HFrEF (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
Although there is an effect of fish oils on important HF outcomes, this recommendation also considers the modest effect size and issues surrounding the lack of standardization of commercial preparations in Canada.
Practical Tip
With most data, the dose of n-3 PUFA is 1 g/d. It is unknown whether higher or lower doses would confer clinical benefit and they are therefore not suggested. Doses greater than 3 g/d are associated with excessive bleeding.
n-3 PUFA therapy might affect measures of anticoagulation. Close monitoring of the international normalized ratio (INR) in patients receiving warfarin after institution of n-3 PUFA is suggested.
There is evidence of significant variability in the content of n-3 PUFA. Patients considering n-3 PUFA should consult with their pharmacist to select a reliable supplement brand that most closely matches formulations shown to be effective in clinical trials.
7.1.1.10 3-Hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitors (statins)
Many patients with HF have coexistent ischemic heart disease; however, these patients were systematically excluded from many of the early landmark statin trials. Two RCTs give insight into the benefit of statins specifically in patient with HF.
The Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) study was an RCT of 5011 patients with HF that compared rosuvastatin 10 mg/d with placebo.[145] There was no difference in the primary end point of cardiovascular mortality, nonfatal MI, or nonfatal stroke. There was an 8% relative reduction in the secondary outcome of cardiovascular hospitalizations, but not HF hospitalizations. Rosuvastatin was well tolerated, with fewer withdrawals from therapy than with placebo. Despite achieving the expected low-density lipoprotein cholesterollowering of rosuvastatin, there was little benefit in this cohort of patients with CAD.[146]
The second trial was the GISSI-HF study; 4574 patients with chronic HF, NYHA class II-IV, irrespective of cause and LVEF, were randomly assigned to rosuvastatin 10 mg/d or placebo, and followed for a median of 3.9 years.[147] There was no difference in the primary end points of time to death, and time to death or admission to hospital for cardiovascular reasons. There was no difference in any other outcomes or subgroups.
Recommendation
We recommend against statins used solely for the indication of HF in the absence of other indications for their use. Statin treatment should be in accordance with primary and secondary prevention guidelines for CVD (Strong Recommendation; High-Quality Evidence).
Practical Tip
Routine statin therapy is unlikely to provide clinical benefit for patients with HF due to nonischemic causes and in the absence of a very high risk of vascular events (such as recent MI, diabetes, and known vascular disease).
In those already receiving statin therapy, it is reasonable to consider statin withdrawal in patients with advanced HF, in polypharmacy where risks outweighs benefits, or when palliative care is an overriding concern.
7.1.1.11 Anticoagulation and antiplatelet therapy
There are no RCTs that evaluate the role of ASA in comparison with placebo in patients with HF. A meta-analysis showed a reduction in serious vascular events, stroke, and coronary events with ASA therapy in secondary prevention trials.[148]
The 2 largest RCTs both compared warfarin with ASA (with or without clopidogrel) rather than placebo. The Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial compared warfarin (INR, 2.5-3), ASA (162 mg) and clopidogrel (75 mg) in patients with HFrEF in sinus rhythm. A total of 1587 patients were followed for a mean of 1.9 years.[149] The study was stopped early secondary to poor recruitment. There was no difference in the primary end point of all-cause mortality, nonfatal MI, or nonfatal stroke in any of the groups. However, there was a reduction in stroke in the warfarin arm compared with the antiplatelet arms, but there was also a higher risk of bleeding in the warfarin group compared with the clopidogrel group. The Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial, the largest trial to date, had similar results with a single comparison arm of ASA 325 mg daily vs warfarin (INR, 2-3.5). A total of 2305 patients were enrolled with a mean follow-up of 42 months.[150] There was no difference in the primary outcome of ischemic stroke, intracerebral hemorrhage, or all-cause mortality, but there was a decrease in ischemic stroke and an increase in major hemorrhage for patients who received warfarin. In a meta analysis of the 4 main RCTs, there was no difference in all-cause mortality, HFrelated hospitalization, or nonfatal MI.[151] There was a decrease in all cause stroke and ischemic stroke and an increase in major bleeding for patients who received warfarin.[152] The ongoing A Randomized, Double-blind, Event-driven, Multicenter Study Comparing the Efficacy and Safety of Rivaroxaban With Placebo for Reducing the Risk of Death, Myocardial Infarction or Stroke in Subjects With Heart Failure and Significant Coronary Artery Disease Following an Episode of Decompensated Heart Failure (COMMANDERHF) trial is testing the additional use of rivaroxaban vs placebo in patients with sinus rhythm, HFrEF, and a recent hospital admission (NCT01877915).
Recommendation
40. We recommend acetylsalicylic acid (ASA) at a dose of between 75 and 162 mg be considered only in patients with HFrEF with clear indications for secondary prevention of atherosclerotic cardiovascular events (Strong Recommendation; High-Quality Evidence).
41. We recommend against routine anticoagulation use in patients with HFrEF who are in sinus rhythm and have no other indication for anticoagulation (Strong Recommendation; High-Quality Evidence).
Anterior ST-elevation myocardial infarction (STEMI) and LV dysfunction has been associated with increased rates of LV thrombus and subsequent thrombotic complications. The rate of LV thrombus associated with anterior STEMI has decreased with more contemporary reperfusion strategies and DAPT. Historical rates range from 3% to 27%, depending on LV function.[153]–[155] However, rates of embolization are much more difficult to quantify. There are no prospective RCTs that address the role of anticoagulation in MI with low EF. A retrospective study of 460 patients done in 2015 evaluated the role of warfarin after primary PCI for anterior STEMI.[156] Warfarin use was at the discretion of the attending physician and 131 patients were discharged receiving warfarin; 99% were discharged receiving DAPT. The rate of death, stroke, need for transfusion, and major bleeding was higher in the warfarin group. Other cohorts have shown similar results.[157] These data should be placed in context with emerging evidence for the use of DAPT as well as non-vitamin K antagonist oral anticoagulants in the setting of an ACS.
Recommendation
42. We recommend against routine anticoagulation after large anterior MI and low EF, in the absence of intracardiac thrombus or other indications for anticoagulation (Weak Recommendation; Low-Quality Evidence).
Values and Preferences
High value is placed on the paucity of compelling evidence supporting efficacy and the potential for harm from bleeding according to the contemporary treatment recommendations with dual antiplatelet therapy (DAPT) post-MI, the emerging efficacy of direct oral anticoagulants after percutaneous coronary intervention (PCI), and the lack of high-quality trial evidence for anticoagulation with warfarin post-MI.
Practical Tip
Anticoagulation may be considered in those with an LV thrombus.
If anticoagulation is used, a duration of 3 months before re-evaluating is reasonable.
Either warfarin or a direct oral anticoagulant could be used for LV thrombus on the basis of the lack of trial evidence and mechanism of action.
7.1.1.12 Anti-inflammatory medications
Several studies have shown that nonsteroidal anti-inflammatory drugs increase the risk of HF. This includes new-onset HF as well as worsening HF outcomes such as hospitalizations and even mortality. There are inconsistent data regarding the safety of individual agents in HF, however most have been associated with negative cardiovascular effects.[158]–[163]
Recommendation
43. We recommend against the use of nonsteroidal anti-inflammatory drugs as well as cyclooxygenase-2 (COX-2) inhibitors in patients with HFrEF (Strong Recommendation; High-Quality Evidence).
Values and Preferences
These agents might cause sodium and water retention, worsen renal function, interact with HF medication (ACEi/ARB), increase cardiovascular events, and worsen HF. Preference is given to reducing drug-related adverse outcomes and should take into account patient preference for pain control and quality of life.
Practical Tip
High doses of ASA might share the same risks as nonsteroidal anti-inflammatory drugs and might aggravate HF, especially in unstable patients.
7.1.1.13 Calcium channel blockers
Most studies on the role of CCBs in HF have shown worsening in HF outcomes.[164]–[167] The Prospective Randomized Amlodipine Survival Evaluation (PRAISE) and PRAISE-2 trials were RCTs that evaluated the effect of amlodipine vs placebo on all-cause mortality and/or cardiovascular hospitalization. There was no difference in either trial in terms of all-cause mortality, cardiovascular death, or hospitalizations.[168],[169] In PRAISE, there was no overall difference between placebo and amlodipine, however, a subgroup analysis showed a reduction on cardiovascular events in patients with a nonischemic etiology of HF.[168] In PRAISE-2, there was no significant difference between amlodipine and placebo in efficacy. The results together suggest caution when using amlodipine.
Recommendation
44. We recommend against the routine use of calcium channel blockers (CCBs) in patients with HFrEF (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
Several RCTs have shown no benefits on, or worsening of, HF outcomes in patients treated with CCBs. Diltiazem, verapamil, nifedipine, and felodipine should be avoided. Amlodipine may be considered for other indications such as persistent hypertension or angina symptoms despite use of GDMT.
Practical Tip
Amlodipine causes dose-related peripheral edema and should be considered when assessing peripheral edema potentially related to HF.
7.1.1.14 Antiarrhythmic drugs
Most anti-arrhythmic drugs (eg, amiodarone) have significant concerns related to their safety profile, especially in HFrEF, and although effective at suppressing atrial or ventricular arrhythmias, might also provoke HF decompensation and cause other adverse effects. When considering these drugs, consultation with an electrophysiologist or individual with appropriate experience and expertise in the use of these drugs is generally advisable.
Recommendation
45. We recommend antiarrhythmic drug therapy in patients with HFrEF only when symptomatic arrhythmias persist despite optimal medical therapy with GDMT, and correction of any ischemia or electrolyte and metabolic abnormalities (Strong Recommendation, Moderate Evidence).
Practical Tip
Only amiodarone has been proven to be acceptable in the HFrEF population.
7.1.2 HFpEF pharmacological treatment
Principles underpinning the pharmacological management of HFpEF include: (1) identification and treatment of underlying etiological factors implicated in the development of HFpEF; (2) identification and treatment of comorbid conditions that might exacerbate the HF syndrome;(3) control of symptoms; and (4) realization of clinically meaningful cardiovascular end points such as HF hospitalization and mortality. There remains a paucity of clinical trial data regarding specific pharmacological therapy in the HFpEF population at this time. Comorbid conditions including other chronic medical diseases are common in the HFpEF population and frequently implicated as triggers for HF decompensation, thus optimal management of these coexistent disorders, including pharmacological and nonpharmacological therapies, should be aggressively pursued.
7.1.2.1 ACEis and ARBs in HFpEF
There is, however, evidence to support the use of ARBs to reduce HF hospitalizations that draws upon secondary end point analysis from the Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM)-Preserved trial.[170] Among 3025 previously hospitalized NYHA class II-IV patients with an LVEF ≥ 40%, candesartan reduced the relative risk of time to first HF hospitalization by 26% compared with placebo. Moreover, a recurrent event analysis of CHARM-Preserved confirmed that this benefit extended to subsequent hospitalizations as well.[171] Reduction in HF hospitalization has also been shown with ACEis, although the evidence is less robust and limited to data from the PEP-CHF study,[172] which included patients 70 years of age or older with an LVEF ≥ 45%. The trial, which had a lower than anticipated event rate and high open-label crossover, did show that perindopril reduced the secondary end point of HF hospitalization by 37% at 1 year although this benefit did not persist over a mean follow-up period of 2.1 years. The I-PRESERVE trial did not show a similar benefit.[173] The ongoing Prospective Comparison of ARNI with ARB Global Outcomes in Heart Failure With Preserved Ejection Fraction (PARAGON-HF) trial is comparing sacubitril-valsartan with valsartan on clinical outcomes in patients with HFpEF (NCT01920711).
7.1.2.2 MRAs in HFpEF
The TOPCAT trial[174] randomized 3445 symptomatic high-risk HFpEF patients, characterized by elevations in NP levels or HF hospitalization within the previous year, to receive spironolactone (mean dose of approximately 25 mg and target dose 45 mg daily) or placebo. Patients were generally older (age older than 50 years) with relatively preserved renal function (eGFR > 30 mL/min) and serum potassium levels (K+ < 5.0 mmol/L). After a mean follow-up period of 3.3 years, there was no difference in the combined primary end point of cardiovascular death, aborted cardiac arrest, or HF hospitalization between groups. When considering the constituent components of the primary end point, only HF hospitalization was decreased in spironolactone treated patients (HR, 0.83; 95% CI, 0.69-0.99). Although elevated potassium levels were more prevalent in the spironolactone arm of the trial (9.1% for placebo vs 18.7% for spironolactone) this did not translate into clinical adverse events including need for dialysis or death due to hyperkalemia. Numerous prespecified and post hoc analyses of the TOPCAT trial have been performed to guide the clinical interpretation and application of these data. Notably, 28.5% of participants were enrolled in the trial on the basis of elevated NP levels. In this group, participants randomized to spironolactone had a 35% reduction in the primary end point compared with those who received placebo. This benefit of spironolactone was not observed among patients who entered the trial on the basis of a previous HF hospitalization. Marked differences in baseline demographic characteristics were observed between inclusion criteria groups; those enrolled on the basis of elevated NP levels were older, had worse renal function at baseline (higher serum creatinine and lower eGFR), and were less likely to be recruited at centres in Russia or Georgia. A significant proportion of patients recruited in the latter region might not have received the assigned study treatment and thus reliable results from TOPCAT might come mainly from the Americas.[175] The observed geographic variation analysis showed a 15% RRR in the primary end point favouring spironolactone in patients enrolled in the Americas vs those enrolled in Russia or Georgia.[86]
Numerous prespecified and post hoc analyses of the TOPCAT trial have been performed to guide the clinical interpretation and application of these data. Notably, 28.5% of participants were enrolled in the trial on the basis of elevated NP levels. In this group, participants randomized to spironolactone had a 35% reduction in the primary end point compared with those who received placebo. This benefit of spironolactone was not observed among patients who entered the trial on the basis of a previous HF hospitalization. Marked differences in baseline demographic characteristics were observed between inclusion criteria groups; those enrolled on the basis of elevated NP levels were older, had worse renal function at baseline (higher serum creatinine and lower eGFR), and were less likely to be recruited at centres in Russia or Georgia. A significant proportion of patients recruited in the latter region might not have received the assigned study treatment and thus reliable results from TOPCAT might come mainly from the Americas.[175] The observed geographic variation analysis showed a 15% RRR in the primary end point favouring spironolactone in patients enrolled in the Americas vs those enrolled in Russia or Georgia.[86]
7.1.2.3 B-Blockers in HFpEF
Although β-blockers provide a plausible physiological mechanism of action for improved outcomes by prolongation of diastolic filling time, reduction of myocardial ischemia, control of hypertension, and arrhythmia prophylaxis, the available quality of evidence and heterogeneity of findings from meta-analyses precludes a firm recommendation for use of this medication class in HFpEF at this time.[176]–[179] As an example, an LVEF subgroup analysis of the Study of the Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure (SENIORS) trial[180] showed a 19% reduction in the combined primary end point of all-cause mortality and cardiovascular hospitalization (HR, 0.81; 95% CI, 0.63-1.04; P for subgroup interaction = 0.043) among study participants with an LVEF ≥ 35% who received nebivolol compared with placebo. However, because of the small effect size of nebivolol in the main SENIORS trial, this analysis lacks power to definitively rule out a significant interaction between outcomes of interest and EF. High dropout rates in the main trial, small sample size, and low event rate in the nonreduced EF group raise further questions about the reproducibility of these findings.
7.1.2.4 Nitrates in HFpEF
Nitrates have been broadly used in patients with established CVD, however, the role of long-acting nitrates in patients with HFpEF is unclear. The Nitrate’s Effect on Activity Tolerance in Heart Failure With Preserved Ejection Fraction (NEATHFpEF) trial[181] enrolled 110 patients to a long-acting nitrate (isosorbide mononitrate 120 mg/d) or placebo into a 6-week crossover trial to test the efficacy and safety of this approach. There was no beneficial effect of nitrates seen in this group on biomarkers, exercise tolerance, activity level, or clinical eventsdand there was a nonsignificant trend toward a lower rate of daily activity for patients who received long-acting nitrates.
Recommendation
46. We suggest candesartan be considered to reduce HF hospitalizations in patients with HFpEF (Weak Recommendation; Moderate-Quality Evidence).
47. We recommend systolic/diastolic hypertension be controlled according to current Canadian Hypertension Education Program hypertension guidelines (2017) (http://www.onlinecjc.ca/article/S0828-282X(17)30110-1/abstract) to prevent and treat HFpEF (Strong Recommendation; High-Quality Evidence).
48. We recommend loop diuretics be used to control symptoms of congestion and peripheral edema (Strong Recommendation; Moderate-Quality Evidence).
49. We suggest that in individuals with HFpEF, serum potassium < 5.0 mmol/L, and an eGFR > 30 mL/min, an MRA like spironolactone should be considered, with close surveillance of serum potassium and creatinine (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendations place a high value on the known etiologic factors for HFpEF and less on known outcome-modifying treatments which, unlike in HFrEF, are still limited.
The MRA recommendation is on the basis of post hoc geographic subgroup analyses of the TOPCAT trial conducted within North and South America mentioned previously.
Practical Tip
Excessive diuretic use can lead to decreased cardiac output and compromise of renal function. Every attempt should be made to use the lowest possible dose of diuretic to achieve and maintain euvolemia.
There is insufficient quality of data to provide strong recommendations regarding statin therapy in HFpEF, so the decision to treat should be customized and on the basis of existing guidelines for primary and secondary prevention of CVD.
After an MRA or ARB is initiated and with a change in dose, serum potassium and creatinine should be monitored in the first week, fourth week, and then fourth month, and whenever clinically indicated.
7.1.3 Implantable cardiac devices
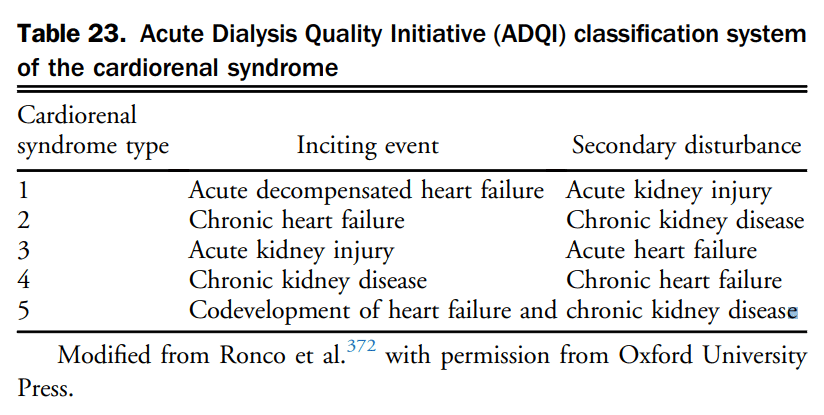
7.1.3.1 Implantable cardioverter-defibrillator therapy
The evidence for the recommendations for implantable cardioverter-defibrillator (ICD) therapy in HF management has been discussed extensively in previous CCS HF guidelines.[182]–[184] Since the publication of these updates, no new indications for ICD therapy have arisen for the general HF population; however, it is worth highlighting some of the most salient points.
7.1.3.1.1 ICD therapy in patients with HF and previous occurrence of sustained ventricular arrhythmia (secondary prevention)
Three large RCTs[185]–[187] (and a subsequent meta-analysis[188]) have compared the use of an ICD with antiarrhythmic drug therapy (primarily amiodarone) in patients with a history of life-threatening ventricular arrhythmias. Most of the patients in these trials had LVSD, and many had symptomatic HF. Although HF symptoms were not often specified as inclusion criteria in many of the trials, most patients had CAD with previous MI or nonischemic cardiomyopathy, with a mean LVEF of 30%-35%. As a primary end point, all-cause mortality was reduced in all studies in the defibrillator-treated patients compared with in the antiarrhythmic drug-treated patients (significantly lower in the Antiarrhythmics Versus Implantable Defibrillators [AVID] study[186] and in the meta-analysis[188]); in the secondary analyses of the studies and the meta-analysis, patients with lower EFs (< 35%), higher NYHA class (classes III or IV), and older age had a higher absolute risk of death and received greater relative and absolute benefits from ICD therapy than did patients without these risk factors. ICDs are the therapy of choice for the prevention of sudden death and all-cause mortality in patients with a history of sustained ventricular tachycardia or ventricular fibrillation, cardiac arrest, or unexplained syncope in the presence of LVSD patients with symptomatic HF, especially with LVEF < 35%, are at particularly high risk of death and stand to receive at least as much benefit as patients not meeting these clinical criteria.
Recommendation
50. We recommend an ICD be implanted in patients with HFrEF and a history of hemodynamically significant or sustained ventricular arrhythmia (secondary prevention) (Strong Recommendation; High-Quality Evidence).
7.1.3.1.2 ICD therapy in patients with HF without a history of sustained ventricular arrhythmia (primary prevention)
On the basis of the available evidence, ICD therapy for primary prevention improves survival in patients with NYHA II-III ischemic and nonischemic HF with EF < 35% and in patients with a previous MI with EF < 30% irrespective of symptom status. In contrast, ICD therapy does not provide any survival benefit early after an MI.[189]–[192]
Landmark clinical trials of ICD therapy in the primary prevention setting selected patients with low LVEF; the most common LVEF cutoff was 35%, although the Multicenter Automatic Defibrillator Implantation Trial II (MADIT II)[190] used < 30%. Although most studies did not specifically select patients with symptomatic HF, the largest study, the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT),[189] included patients with current NYHA class II or III symptoms, and a history of HF for more than 3 months.
When considering the risk of sudden death and potential benefit from an ICD, the contribution of systolic dysfunction per se vs HF symptoms has not been fully defined. Secondary analyses of most studies have indicated that the absolute risk of sudden death, as well as the relative and absolute mortality benefits of an ICD, was greater for patients with lower LVEF (< 30%).
The contribution of HF symptoms (as distinct from LVEF) to the absolute and relative benefit of an ICD remains unclear. In MADIT II, in which patients with NYHA class I, II, or III could be enrolled, patients with greater symptoms of HF appeared to derive relatively greater benefit from an ICD.[190] In contrast, patients in SCD-HeFT with class III HF appeared to have a smaller RRR) than those with NYHA class II symptoms.[189]
Results on the basis of 12 RCTs (8516 patients) and 76 observational studies (96,951 patients), showed that ICD therapy was associated with a 1.2% implantation mortality and a total 3.5% annual likelihood of complications including device malfunction, lead problems, or infections. There was a 4%-20% range of annual inappropriate discharge rates.[193] It is important to note that in RCTs that specifically selected patients early (< 40 days) after a MI, there was no significant benefit from the ICD compared with control therapy.[191],[192]
Finally, recent evidence has called into question the benefit of ICDs for primary prevention in patients with nonischemic cardiomyopathy. Historically, the data supporting ICD use in this population have been less robust, and guideline recommendations have been largely on the basis of older systematic reviews and RCTs, including the Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE)[194] and SCD-HeFT trials, that have shown a reduction in sudden death with ICDs in patients with nonischemic cardiomyopathy. However, the recently published Danish Study to Assess the Efficacy of ICDs in Patients with Nonischemic Systolic Heart Failure on Mortality (DANISH) trial randomized 1116 patients with nonischemic HF, LVEF ≤ 35%, NYHA II-IV symptoms, and elevated NT-proBNP to ICD vs no ICD in addition to contemporary HF therapy.[195] In this study, there was no difference between groups with respect to the primary outcome of all-cause mortality after a median follow-up of approximately 5.5 years. Importantly, ICD use was associated with a reduction in sudden cardiac death (SCD) in the overall study population, and a reduction in all-cause mortality in the subgroup of patients younger than 68 years of age. DANISH also included a very high proportion of patients treated with CRT (58%), which might have offset some of the benefits of ICD therapy. Indeed, the degree of benefit of ICDs in the setting of nonischemic cardiomyopathy is the subject of ongoing investigation; in an updated meta-analysis of primary prevention ICDs in nonischemic cardiomyopathy that included the DANISH trial a significant 23% risk reduction in all-cause mortality favouring ICD use was reported.[196] In balance, the weight of evidence appears to favour the use of ICDs for primary prevention in nonischemic HF, however, recent data highlight the need to individualize decision-making and recommendations around ICDs, and further informs the discussion between clinicians and patients regarding the anticipated effects of this therapy.
The assessment of LVEF for ICD consideration should be performed after titration and optimization of medical therapy. It is reasonable to evaluate response to therapy and LV function at least 3 months after titration of medical therapy. In addition to cardiac status, consideration of other comorbid conditions, patient desires, and goals of therapy are essential components in the assessment for prescription of ICD therapy in this group of patients. In addition, close collaboration between the referring or HF physician and the arrhythmia specialist is essential, not only in the initial assessment of these patients, but in their follow-up. Additional considerations and related guidance is available in the CCS/Canadian Heart Rhythm Society 2016 ICD guidelines.[197]


Recommendation
51. We recommend consideration of primary ICD therapy in patients with:
i. Ischemic cardiomyopathy, NYHA class II-III, EF ≤ 35%, measured at least 1 month post MI, and at least 3 months post coronary revascularization procedure (Strong Recommendation; High-Quality Evidence); or
ii. Ischemic cardiomyopathy, NYHA class I, and an EF ≤ 30% at least 1 month post MI, and at least 3 months post coronary revascularization procedure (Strong Recommendation; High-Quality Evidence); or
iii. Nonischemic cardiomyopathy, NYHA class II-III, EF ≤ 35%, measured at least 3 months after titration and optimization of GDMT (Strong Recommendation; High-Quality Evidence).
52. We recommend against ICD implantation in patients with NYHA class IV symptoms who are not expected to improve with any further therapy and who are not candidates for cardiac transplantation or mechanical circulatory support (MCS) (Strong Recommendation; Moderate-Quality Evidence).
7.1.3.2 Device considerations in patients with HF after cardiac surgery
The rationale and evidence supporting the use of devices, ICD and CRT, in patients with HF and reduced EF have been addressed in detail in previous HF and CRT guideline updates.[184],[198]
Although no studies to date have directly assessed the optimal timing of ICD implantation in the setting of ischemic cardiomyopathy, evidence from primary prevention trials suggests that ICDs do not confer an overall mortality benefit when implanted during, or immediately after, an acute event or revascularization.[191] The Coronary Artery Bypass Graft (CABG) Patch trial was designed to assess whether an ICD is associated with additional survival benefit in patients at high risk for SCD who undergo coronary artery bypass graft (CABG) surgery.[199] The negative findings in this trial were essentially mirrored in other studies of ICD after acute MI and reinforce the role of ICD therapy to be one for chronic LV dysfunction.[191],[192],[200]
After revascularization, the risk of SCD continues over time,[201] while systolic function might not improve substantially,[202] posing a challenge in defining the optimal timing for ICD therapy.
Similarly, the optimal timing for CRT implantation in suitable candidates with ischemic cardiomyopathy has not been well defined. Key clinical trials reporting a mortality benefit with CRT excluded patients with a recent (1-6 months) MI or revascularization procedure.[203]–[205] However, data from observational studies provide a rationale for considering epicardial LV lead placement at the time of CABG surgery in patients who might otherwise have an indication for CRT. Transvenous LV lead delivery via the coronary sinus is technically not feasible in approximately 10% of cases[206]; surgical lead placement can overcome anatomical limitations imposed by the coronary sinus, with acceptable long-term lead performance and rates of clinical response similar to conventional transvenous implantation.[207] Additionally, surgical revascularization might not have any effect on dyssynchrony, which is associated with a worse prognosis.[208] Data from one RCT[209] suggest that CRT using an epicardial lead implanted concomitantly with CABG is associated with improved systolic function and survival compared with CABG alone in patients with poor systolic function and evidence of preoperative device candidacy. Therefore, epicardial LV lead placement might be considered in selected patients who undergo surgical revascularization for ischemic cardiomyopathy who are likely to remain candidates for CRT after surgery.
Perioperative management of existing devices remains an important component of care; in keeping with existing guidelines, which state device deactivation is necessary before any procedure in which electrocautery, or potential for electrical interference with the device might occur.[210] Postoperatively, re-establishment of appropriate device threshold determination and programming are recommended.
Recommendation
53. We recommend that after successful cardiac surgery, patients with HF undergo assessment for implantable cardiac devices within 3-6 months of optimal treatment (Strong Recommendation; High-Quality Evidence).
54. We recommend that patients with implantable cardiac devices in situ should be evaluated for programming changes before surgery and again after surgery, in accordance with existing CCS recommendations197 (Strong Recommendation; Low-Quality Evidence).
Practical Tip
During surgical revascularization, consideration can be given to implantation of epicardial LV leads to facilitate biventricular pacing in eligible patients who might be candidates for CRT, especially if the coronary sinus anatomy is known to be unfavourable for lead placement.
7.1.3.2.1 ICD therapy to prevent sudden death in patients with hypertrophic cardiomyopathy
Although a detailed review of specific cardiomyopathies is beyond the scope of this document, prevention of SCD in patients with an established diagnosis of HCM in particular has been an area of active study discussed elsewhere.[211],[212]
Cardiovascular death, frequently due to sudden death, is a well-recognized complication of HCM, at approximately 1%-2% per year.[211],[212] Well established clinical risk factors for sudden death include: previous cardiac arrest, ventricular fibrillation, or sustained ventricular tachycardia, a history of sudden death in close relatives (particularly at a young age), a history of unexplained syncope, LV wall thickness ≥ 30 mm, nonsustained ventricular tachycardia (≥ 3 beats at ≥ 120 bpm) on Holter monitoring, and blunted BP response to exercise.[211] Although patients with multiple risk factors are at higher risk, the relative weight or importance of individual risk factors for clinical decision-making in the primary prevention setting remains the subject of ongoing study. Current guideline-based risk stratification approaches appear to have limited ability to discriminate high- vs lower-risk patients.[213]
More recently, the HCM Risk-SCD Prediction Model[214] is a retrospectively derived risk score that provides an absolute estimate of 5-year risk of sudden death. Attempts at validating the HCM Risk-SCD Prediction Model have yielded conflicting results in different patient populations and in different practice settings.[215],[216] It is therefore important to recognize that current approaches to risk stratification for SCD in HCM have limitations; patient factors and other markers of risk (including specific genetic mutations, identification of LGE on CMR imaging, for example) might modify the assessment of risk in an individual patient.
There is no evidence that drug therapy reduces the risk of sudden death, even in high-risk patients. An ICD is indicated for patients with HCM who survive a cardiac arrest or have had sustained ventricular tachycardia. Although there are no prospective RCTs to guide therapy for primary prevention, there is consensus that consideration should be given to implantation of an ICD in patients with multiple high-risk factors and in patients whose estimated absolute risk (of SCD) is high.[211],[212] Patients with a single high-risk factor should be individually assessed for ICD implantation, including a discussion of the level of risk acceptable to the individual and potential adverse effects with an ICD, such as inappropriate ICD discharges, lead complications, and infection.
Recommendation
55. We recommend patients with hypertrophic cardiomyopathy (HCM) who survive a cardiac arrest should be offered an ICD (Strong Recommendation; Moderate-Quality Evidence).
56. We recommend patients with HCM who have sustained ventricular tachycardia should be considered for an ICD (Strong Recommendation; ModerateQuality Evidence).
57. We suggest an estimate of risk for SCD in patients with HCM should be determined on the basis of validated risk scores and/or the presence of one or more high-risk clinical factors to select appropriate candidates for primary prevention ICD therapy (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendations place great value on the prevention of SCD in patients perceived to be at high risk from observational studies. Primary prevention ICD recommendations in this population place significant weight on individualizing risk assessment whenever possible by clinicians/centres with significant experience in HCM, taking into consideration the potential for device complications.
Practical Tip
Emerging risk factors for SCD, including late gadolinium enhancement (LGE) on CMR imaging, specific genetic mutations, and electrocardiographic features might be considered to modify estimates of risk on an individual basis by clinicians/centres with significant experience managing patients with HCM.
7.1.3.3 CRT
Despite optimization of GDMT, LV systolic dysfunction and HF symptoms persist for many patients. Commonly, these patients have conduction delay, typically expressed as an LBBB pattern that is associated with cardiac mechanical dyssynchrony. This compromises ventricular function and is associated with poor prognosis. CRT attempts to synchronize the activation of the ventricles as well as the atrioventricular activation sequence, which leads to short-term and long-term improvements in overall LV function.
The publication of landmark trials and analyses mandated the revision of the earlier recommendations to include patients with mild HF symptoms and to place more emphasis on QRS morphology and duration, and the importance of sinus rhythm in the selection of CRT patients.[183],[184] Further systematic reviews and long-term follow-up data from RCTs have confirmed the benefits of CRT and helped refine the selection of ideal candidates for this therapy. The updated recommendations have been harmonized with the comprehensive CCS guidelines on the use of cardiac resynchronization therapy: evidence and patient selection.[198]
Several landmark studies have shown the effectiveness of CRT to improve morbidity and mortality in selected patients with HFrEF. Al-Majed et al. performed a systematic review of RCTs[217] that included 25 studies of 9082 patients with LVEF ≤ 40% and compared CRT vs usual care or ICD or RV pacing alone. Pooled data from all studies showed that CRT reduced mortality by 19%. Analysis of outcomes according to NYHA functional class revealed a 17% reduction in mortality and 29% reduction in HF hospitalization among patients with NYHA I-II symptoms. Similarly, there was a 20% reduction in mortality and 35% reduction in HF hospitalization among patients with NYHA III-IV symptoms. CRT was associated with a 94.4% implantation success rate, 3.2% risk of mechanical complications, 6.2% risk of lead complications, and peri-implant mortality of 0.3%. Results of other systematic reviews, including individual patient meta-analyses[218]–[222] have yielded similar findings, suggesting that CRT improves survival and HF hospitalization in a spectrum of HFrEF patients with mild or severe HF symptoms. Finally, since the publication of these reviews, the long-term follow-up of the Multicenter Automatic Defibrillator Implantation Trial With Cardiac Resynchronization Therapy (MADIT-CRT) study has been published.[223] In this trial, 1818 patients with NYHA I and II symptoms, LVEF < 30%, and QRS ≥ 130 ms were randomized to CRT defibrillators (CRT-D) vs ICD only and median follow-up was 5.6 years. Among patients with LBBB, there was a 41% relative reduction in mortality, and among patients with right bundle branch block, a 57% relative increase in mortality. This analysis confirms the long-term mortality benefit in patients with mild HF, reduced EF, and LBBB beyond the benefits in morbidity reported in the primary trial.
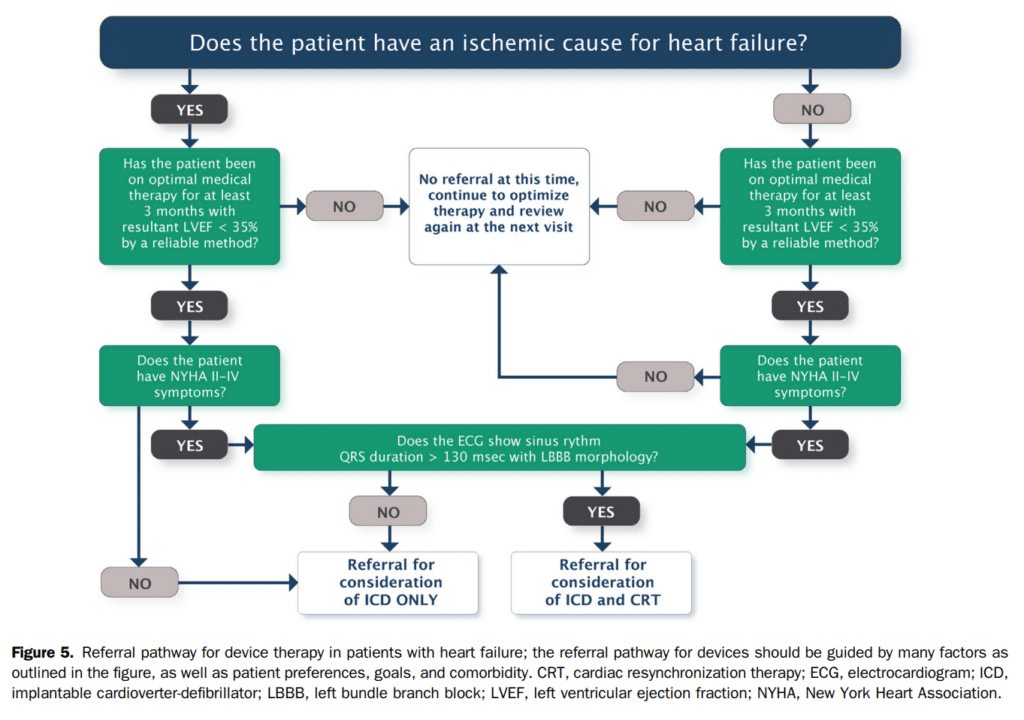
An important and consistent finding in systematic reviews and in subgroup analyses of RCTs is that the benefits of CRT are greatest for patients with a broader QRS, typically defined as QRS duration > 150 ms, and for patients with a typical LBBB QRS morphology.[223]–[227] It remains unclear whether patients with a relatively narrow QRS (120-150 ms) or those with non-LBBB derive any benefit from CRT, or whether other clinical factors could help select potentially appropriate candidates among these subgroups. Last, the interaction between QRS duration and morphology and its importance for CRT also warrants further evaluation; it is conceivable that patients with very broad QRS and non-LBBB morphology might derive some magnitude of benefit from CRT.[226] Current recommendations for CRT candidate selection are therefore on the basis of the characteristics of patients included in landmark studies and on the clinical characteristics of patients shown to derive significant benefit from CRT on the basis of the totality of available data (Fig. 5).
7.1.3.3.1 CRT in patients with AF
Most RCTs that evaluated CRT included only patients in sinus rhythm, and relatively few patients with permanent AF were included in prospective randomized studies of CRT efficacy. Achieving atrioventricular synchronization is an important goal for most patients in sinus rhythm who undergo CRT, and it is therefore unclear whether patients with permanent AF who are otherwise candidates derive any meaningful benefit from CRT. To date, the Resynchronization for Ambulatory Heart Failure Trial (RAFT) is the largest RCT to include patients with AF with intact AV nodal conduction. In a substudy of RAFT[228] the effect of CRT in patients with permanent AF was evaluated; 114 patients were randomized to CRT-D and 115 patients were randomized to ICD alone and LVEF (22.9% vs 22.3%) and QRS duration (151.0 ms vs 153.4 ms) were similar between groups. In this study, CRT was not associated with improvements in the combined end point of death or HF hospitalization or cardiovascular death, HF hospitalizations, change in 6-minute walk, or quality of life. A major limitation of this analysis is that only one-third of patients achieved biventricular pacing 95%, and only 1 patient underwent AV nodal ablation to effect 100% biventricular pacing.
Indeed, observational data strongly suggest that outcomes in AF patients who receive CRT are associated with the degree of biventricular pacing achieved, and that differential effects in survival might be seen when biventricular pacing achieved is < 98% vs > 98%.[229] A meta-analysis of observational studies of AV node ablation vs pharmacologic rate control in AF and CRT (1256 patients; 644 with AV node ablation, 798 without AV node ablation) suggested that AV nodal ablation is associated with a higher degree of biventricular pacing (100% vs 82%-96%), reduced mortality, and lower rates of CRT nonresponse compared with pharmacologic rate control.[230] Ongoing prospective RCTs, including the multicentre Resynchronization/Defibrillation for Ambulatory Heart Failure Trial in Patients With Permanent AF (RAFT-PermAF) should help refine the role of CRT in patients with AF who would otherwise be suitable candidates.
7.1.3.3.2 CRT in patients with RV pacing and reduced EF
The use of CRT in patients with LVSD who require permanent ventricular pacing, or in patients with suspected RV pacing-induced HF has been investigated.[231]–[233] Although the prognostic significance of RV pacing-induced dyssynchrony vs intrinsic LBBB-related dyssynchrony is uncertain, a subgroup of patients with frequent RV pacing will experience worsening of LV function, particularly in the setting of abnormal LVEF and HF at baseline. The results of these studies suggested that CRT in this clinical situation improves LV function, symptoms, and exercise capacity.[231] To address this issue further, the Biventricular Versus RV Pacing in Patients with LVSD and Atrioventricular Block (BLOCK HF) study randomized 691 patients with LVEF ≤ 50% and heart block to CRT vs RV pacing (with an ICD or pacemaker as indicated).[234] Patients in this study had a mean LVEF of 40%, and > 80% had NYHA class II or III symptoms. After a mean follow-up of 37 months, CRT was associated with fewer primary outcome events including the composite of death, urgent care visit for intravenous (I.V.) HF therapy, or an increase in LV end systolic volume index ≥ 15% (HR, 0.74; 95% CI, 0.60-0.90). The benefits observed with CRT were driven by reductions in HF events. Notably, pacing percentage in both study groups was > 97% and serious adverse events occurred in 14% of patients, mainly related to lead complications. Overall, it appears that patients similar to those included in the BLOCK HF study derive significant benefits with CRT compared with RV only pacing with respect to HF events, but the potential for procedural complications needs to be considered carefully for individual patients.
7.1.3.3.3 CRT in patients with narrow QRS
Compared with ICD alone, CRT has not been associated with improvements in mortality or HF hospitalization, and there is a suggestion of increased harm with CRT in some studies.[235]–[239]
Recommendation
58. We recommend CRT for patients in sinus rhythm with NYHA class II, III, or ambulatory class IV HF despite optimal medical therapy, a LVEF ≤ 35%, and QRS duration ≥ 130 ms with left bundle branch block (LBBB) (Strong Recommendation; High-Quality Evidence).
59. We suggest that CRT may be considered for patients in sinus rhythm with NYHA class II, III, or ambulatory class IV HF despite optimal medical therapy, a LVEF ≤ 35%, and QRS duration ≥ 150 ms with non-LBBB (Weak Recommendation; Low-Quality Evidence).
Practical Tip
There is no clear evidence of benefit with CRT among patients with QRS durations < 150 ms because of non-LBBB conduction.
Recommendation
60. We suggest that CRT may be considered for patients in permanent AF who can expect to achieve close to 100% pacing and are otherwise suitable for this therapy (Weak Recommendation; Low-Quality Evidence).
Practical Tip
It is important to ensure that the amount of biventricular pacing approaches 100% where possible. AV junctional ablation might be necessary to achieve sufficient biventricular pacing.
Recommendation
61. We suggest that CRT might be considered for patients who require chronic right ventricular (RV) pacing in the setting of HF symptoms and reduced LVEF (Weak Recommendation; Moderate-Quality Evidence).
62. We recommend CRT not be used for patients with QRS < 130 ms, irrespective of HF symptoms, LVEF, or the presence or absence of mechanical dyssynchrony shown on current imaging techniques (Strong Recommendation; Moderate-Quality Evidence).
63. We recommend the addition of ICD therapy be considered for patients referred for CRT who meet primary ICD requirements (Strong Recommendation; High-Quality Evidence).
Values and Preferences
These recommendations place a value on the benefit of CRT in patient groups included in the landmark RCTs and high-quality systematic reviews, and less value on post hoc subgroup analyses from clinical trials. On the basis of the available evidence, there is insufficient evidence to recommend CRT in patients with NYHA class I status or in hospitalized NYHA class IV patients. Patients with a QRS duration ≥ 150 ms are universally more likely to benefit from CRT than patients with less QRS prolongation. CRT pacemaker therapy should also be considered in patients who are not candidates for ICD therapy such as those with a limited life expectancy because of significant comorbidities, and in patients who decline to receive an ICD.
7.1.4 Advanced HF management strategies
Although the term, advanced HF has many definitions, to guide clinicians as to which patients should be considered for advanced HF management (such as but not limited to cardiac transplantation, MCS, or palliative care) the following is a general guide. Cardiac transplantation is well established in Canada and further guidance is available at https://ccs.ca/en/cctn-home. Cardiac transplantation assessment is typically done by a multispecialty, multidisciplinary team in a specialized setting, using Canadian and international guidance for appropriate workup and eligibility.
Patients with advanced HF to be considered for advanced HF management strategies include those who, despite optimal treatment, continue to exhibit progressive/persistent NYHA III or IV HF symptoms and accompanied by more than one of the following:
- LVEF < 25% and, if measured, peak exercise oxygen consumption < 14 mL/kg/min (or less than 50% predicted).
- Evidence of progressive end organ dysfunction due to reduced perfusion and not to inadequate ventricular filling pressures.
- Recurrent HF hospitalizations (≥ 2 in 12 months) not due to a clearly reversible cause.
- Need to progressively reduce or eliminate evidencebased HF therapies such as ACEis, MRAs, or β-blockers, because of circulatory-renal limitations such as renal insufficiency or symptomatic hypotension.
- Diuretic refractoriness associated with worsening renal function.
- Requirement for inotropic support for symptomatic relief or to maintain end organ function.
- Worsening right HF (RHF) and secondary pulmonary hypertension.
- Six-minute walk distance < 300 m. Increased 1-year mortality
- (eg, > 20%-25%) predicted by HF risk scores
- Progressive renal or hepatic end organ dysfunction.
- Persistent hyponatremia (serum sodium < 134 mEq/L).
- Cardiac cachexia.
- Inability to perform activities of daily living.
It should be noted that most patients will have a number of the listed criteria and there is no single criterion that determines candidacy for cardiac transplantation, MCS, or palliative care. Patient preferences should be incorporated into the decision process when assessing further choices.
7.1.5 MCS
7.1.5.1 What is mechanical circulatory support?
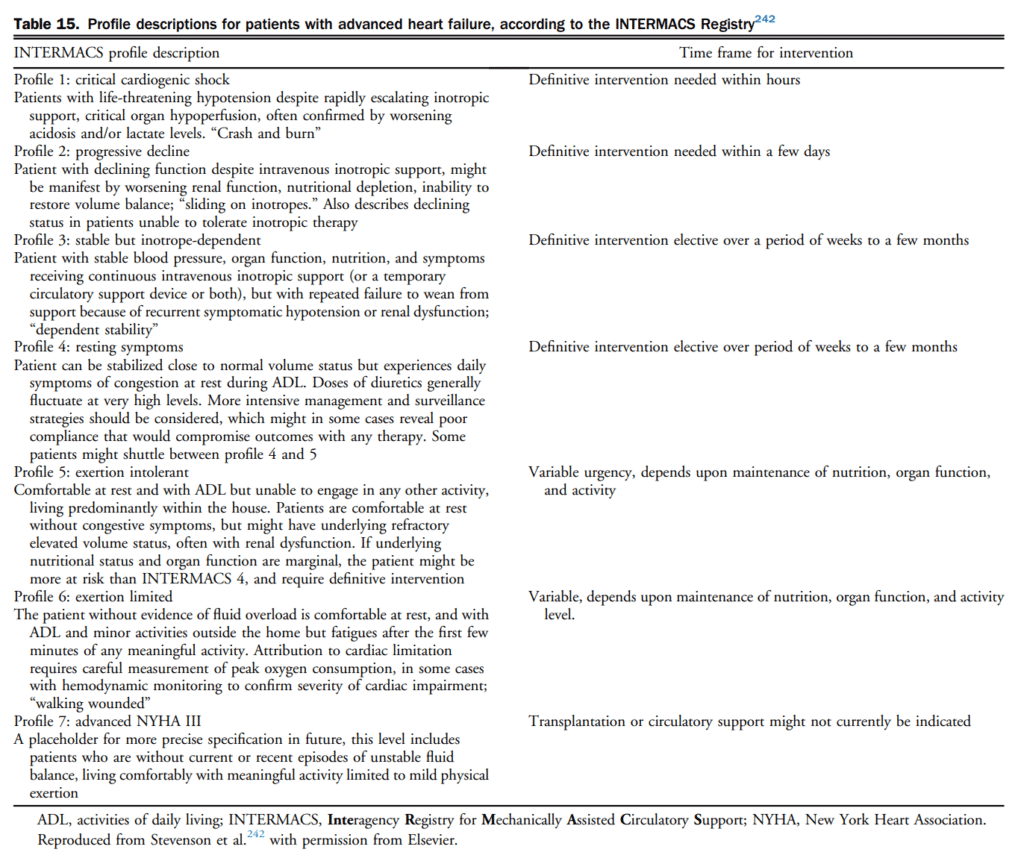
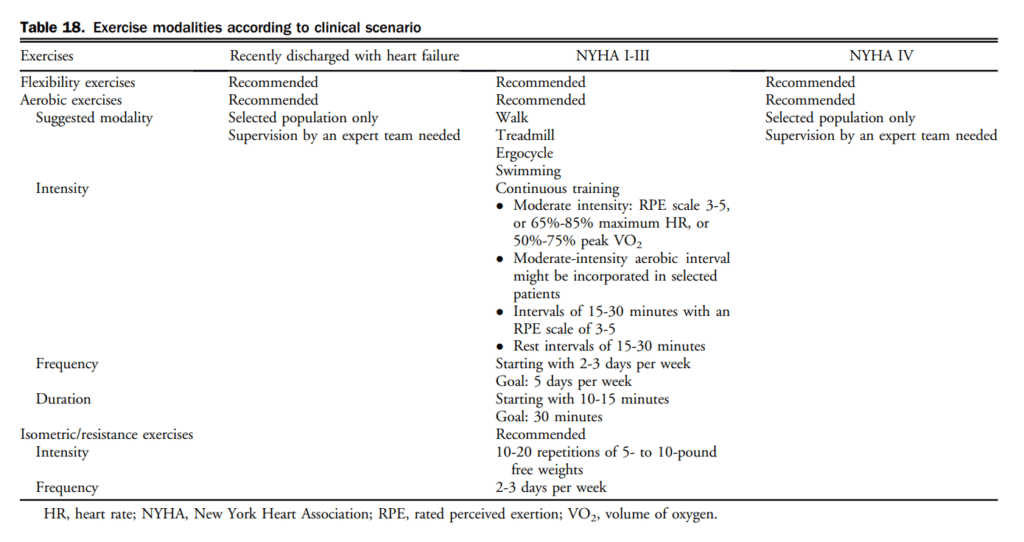
MCS is a group of technologies that increase forward cardiac output in patients.[240] MCS therapies consist of ventricular assist devices that augment or replace the ventricle. They may be used to assist the right ventricle, left ventricle, or both ventricles.[241] The choice depends on the clinical presentation, and can be divided into 2 categories-temporary circulatory support and long-term devices. Details of the purpose of MCS, the decision process, description of patient profiles, management, and other issues are outlined in sections 7.1.5.2-7.1.5.7 of the Supplementary Material, and in Tables 15-17.
Practical Tip (choice of temporary MCS)
Vasopressors and positive inotropic agents remain the first lines of treatment, but frequently offer inadequate support; then, the use of percutaneous MCS in severe, refractory cardiogenic shock should be considered early in a patient’s clinical course.
The choice of which MCS device to use is on the basis of many factors, including patient characteristics, the degree of desired hemodynamic support, operator abilities, and institutional resources.
In general, there is a continuum of increasing hemodynamic support from the intra-aortic balloon pump (IABP) to the Impella 2.5 and CP devices to the TandemHeart and VA-ECMO.
The choice of which device to use is multifactorial, on the basis of patient characteristics, operator ability, and the degree of hemodynamic support desired.
These devices are best managed with a care team approach that includes an advanced HF cardiologist.
In general, patients with HF are potentially candidates for MCS if they fulfil the advanced HF criteria mentioned previously.
Practical Tip (MCS-performing centres)
Cardiac centres that perform MCS should have adequate manpower and resources for support of patients requiring MCS support. These include:
- An identified and adequately trained multidisciplinary MCS team;
- Access to the full array of medical and surgical consultative support, and institutional administrative and financial support; and
- Expertise in MCS implantation, follow-up, and explantation.
Recommendation
64. We recommend that patients with either acute severe or chronic advanced HF and with an otherwise good life expectancy be referred to a fully equipped cardiac centre for assessment and management by a team with expertise in the treatment of severe HF, including MCS (Strong Recommendation; Moderate-Quality Evidence).
65. We recommend MCS be considered for patients who are listed for cardiac transplantation and who deteriorate or are otherwise not likely to survive until a suitable donor organ is found, including those for whom a long wait is expected (Strong Recommendation; High-Quality Evidence).
66. We recommend that MCS be considered for patients for whom there is a contraindication for cardiac transplantation but might, via MCS, be rendered transplantation-eligible (Strong Recommendation; Low-Quality Evidence).
67. We recommend that patients in cardiogenic shock be considered for temporary MCS to afford an opportunity for evaluation for long-term options (Strong Recommendation; Moderate-Quality Evidence).
Practical Tip
Extracorporeal circulatory membrane oxygenator or other mechanical circulatory temporary devices should be preferred over the IABP except if the patient is suffering an acute ischemic event, because the increase in cardiac output offered by the IABP is usually minimal.
Recommendation
68. We recommend permanent MCS be considered for highly selected transplantation-ineligible patients (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation places a high value on the potential variability of patient preference as well as the need to interact with the patient to ensure the choice reflects the patient’s values, with less value on the effectiveness of therapy.
69. We recommend that institutions providing MCS therapy develop a policy regarding destination therapy within the conventions, resources, and philosophy of care of their organization (Strong Recommendation; Low-Quality Evidence).
70. We recommend that ambulatory patients with MCS therapy who are discharged from hospital and who have had minimal HF symptoms or ventricular arrhythmias for a period of at least 2 months be considered candidates for operation of a personal motor vehicle for a period not exceeding two-thirds of the known battery charge time (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
An objective assessment of the disease severity and prognosis for an individual patient using a validated scoring system is recommended. If the expected mortality is higher than the procedural risk of advanced HF therapies, these patients should be considered for referral, provided they have a good life expectancy otherwise.
Practical Tip
The timing of discussions should strongly consider the high mortality rate in the year after a first HF hospitalization. A surrogate decision-maker should be identified early and regularly participate in these discussions.
7.1.6 Exercise and rehabilitation
Exercise intolerance is recognized as a hallmark of HF. It is now understood that exercise intolerance in HF has a multifactorial etiology and that parameters such as intracardiac filling pressures and LVEF might not be reliable predictors of exercise capacity. Changes in the periphery and LV function are both important determinants of exercise capacity. Therefore, it is rational that exercise training could potentially benefit patients with HF.
There have been several systematic reviews and metaanalyses that show the benefits of exercise training for patients with HF.[243]–[245] There has been one large RCT that has shown the benefits of exercise training.[246] In the Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study 2331 medically stable patients with HF were randomized to regular exercise training or usual care. There was a nonsignificant 7% relative reduction in the primary outcome of all-cause mortality or hospitalization. After adjusting for the key covariates of duration of the cardiopulmonary exercise test, LVEF, Beck Depression Inventory II score, and history of AF or flutter along with HF etiology exercise training was found to produce an 11% relative reduction of all-cause mortality or all-cause hospitalization. There was no difference in adverse events between the 2 groups. A systematic review examined the effectiveness of exercise-based rehabilitation in 33 trials with 4740 patients with predominately HFrEF.[243] There was no reduction in allcause mortality with up to 1 year of follow-up, and a trend to reduction in all-cause mortality in trials with more than 1 year of follow-up (6 trials; 2845 participants; relative risk, 0.88; 95% CI, 0.75-1.02). Exercise training did reduce all-cause hospitalization and HF hospitalizations. Importantly, no safety concerns were raised in any of the studies.[245]–[248]
Exercise training results in increases of exercise capacity.[249],[250] A meta-analysis of studies that directly measured peak volume of oxygen (VO2) reported an average 17% improvement in peak VO2.[245] The evidence for quality of life improvements in patients with HF exposed to exercise training is further supported by multiple studies.[245],[251] Although there have been a great variety of types of exercise training strategies most have involved moderate to vigorous exercise as was prescribed in the HF-ACTION study.[250]
Although the dose of physical activity that conveys cardiovascular and other health benefits is difficult to categorically quantify, there is support for as little as 15 minutes per day of moderate-intensity physical activity with further dose response thereafter.[252] Piepoli et al.[253] have provided an overview about the practical approaches to exercise training for patients with HF.
The role of exercise training in HFpEF patients is less well established. However, the available data suggest exercise training has benefits that include improvements in exercise capacity and quality of life.[244],[254]–[256] Studies have also shown that exercise training can take place in patients with ICD or CRT therapy. These studies have shown that properly prescribed and monitored exercise training can safely result in improvements in exercise capacity in patients with an ICD and/or CRT therapy (Table 18).[257],[258]
Recommendation
71. We recommend regular exercise to improve exercise capacity, symptoms, and quality of life in all HF patients (Strong Recommendation; Moderate-Quality Evidence).
72. We recommend regular exercise in HF patients with reduced EF to decrease hospital admissions (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendations have placed a high value on regular exercise and not emphasized structured exercise training because it is recognized that not all patients will be able to participate in a structured exercise training program because of patient preferences or availability of resources.
Practical Tip
It is important to individualize the exercise training for each patient, with the more deconditioned patients starting at a lower training intensity and with shorter sessions.
7.1.7 Important nonpharmacological and nondevice management options
The basic treatment of HF has included advice on dietary salt and fluid restriction. The evidence to support these concepts is scarce and some evidence suggests the opposite of current clinical practice.[259]–[262]
Dietary sodium consumption for patients with HF remains controversial. Several cohort studies and 1 RCT suggest that lower dietary sodium intake is associated with better clinical outcomes.[260],[262],[263] Other studies suggest that the combination of dietary sodium restriction and high-dose diuretics with or without saline infusions can be deleterious, summarized by Gupta et al.[264] An ongoing RCT will provide guidance on this topic (NCT02012179).
Severe fluid restriction is not only difficult to maintain but could also have deleterious effects without additional benefit. Three hospital-based RCTs in this area suggest no additional benefit of the combination of fluid restriction with or without sodium restriction.[265]–[267] Special consideration for severe water restriction should be considered for hyponatremic patients with hypervolemia and applied sparingly. High-quality data are lacking on this topic, and no high-quality evidence exists in the ambulatory care environment. Thus, for patients admitted to hospital or as outpatients, allowing liberal fluid intake is reasonable.
Alcohol consumption should be limited for all patients with HF, and if it is believed to be responsible and/or contributing to the syndrome it should be avoided altogether because there is a dose-dependent effect and individual susceptibility to the deleterious effects of alcohol.[268]
Because smoking has been linked to the progression of CAD all attempts should be done to promote smoking cessation, even if HF is not present. Nicotine replacement therapy and/or other smoking cessation therapies are acceptable for most patients with HF. There is limited evidence of the effects of e-cigarettes (“vaping”) or medical marijuana for patients with established HF.
Recommendation
73. We suggest that patients with HF should restrict their dietary salt intake to between 2 g/d and 3 g/d (Weak Recommendation; Low-Quality Evidence).
Practical Tip
The optimal quantity of salt in the diet is still a subject of debate. The amount should be adapted to the clinical situation, the severity of symptoms, and baseline consumption without interfering with other nutritional content.
Recommendation
74. We suggest daily morning weight should be monitored in patients with HF with fluid retention or congestion that is not easily controlled with diuretics, or in patients with significant renal dysfunction (Weak Recommendation; Low-Quality Evidence).
Practical Tip
Weight should be closely monitored for unstable or frail patients. Any rapid weight gain (ie, > 1.5 or 2 kg) should prompt a rapid medical visit. Weight loss should also be addressed medically.
Recommendation
75. We suggest that restriction of daily fluid intake to approximately 2 L/d should be considered for patients with fluid retention or congestion that is not easily controlled with diuretics (Weak Recommendation; Low-Quality Evidence).
Practical Tip
The appropriate quantity of fluid intake is a subject of debate. Strict limits should be imposed when there is clear fluid overload or demonstrated sensitivity to fluid intake.
Severely limiting daily fluid intake to < 1.5 L might have adverse consequences on nutrition, renal function, and quality of life without known additional benefit and should be applied selectively.
Special consideration for hyponatremic patients should be applied.
Alcohol intake should be avoided if it is a precipitating or contributing factor.
Patients should quit smoking and a referral for counselling should be offered.
7.2 Cardiovascular comorbidities
7.2.1 Atrial fibrillation
AF and HF share common risk factors and frequently coexist.[270],[271] Up to 50% of patients with HF might develop AF; the reported prevalence rates of AF among HF cohorts varies significantly and is largely dependent on the clinical setting (acute vs community), the extent of LV dysfunction, NYHA functional class, and the use of background HF therapies.[272]
The presence of AF is associated with a worse prognosis in terms of overall survival[273],[274] as well as risk of stroke.[275] AF might also exacerbate the HF syndrome through a number of mechanisms including: (1) decreased cardiac output secondary to loss of atrial systole; (2) increased myocardial oxygen consumption and decreased coronary perfusion during periods of rapid ventricular response; (3) neurohormonal activation; and (4) the development of tachycardia-induced cardiomyopathy.[276] Further details pertaining to primary prevention, rate and rhythm control, and anticoagulation can be found in sections 7.2.1.1-7.2.1.3 of the Supplementary Material.
Recommendation
76. We recommend in patients with HF and AF that the ventricular rate be controlled at rest and during exercise (Strong Recommendation; Moderate-Quality Evidence).
77. We recommend β-blockers for rate control particularly in those with HFrEF (Strong Recommendation; Moderate-Quality Evidence).
78. We recommend rate-limiting CCBs be considered for rate control in HFpEF (Weak Recommendation; Low-Quality Evidence).
79. We recommend the use of antiarrhythmic therapy to achieve and maintain sinus rhythm; if rhythm control is indicated, it should be restricted to amiodarone (Strong Recommendation; Moderate-Quality Evidence).
80. We recommend the additional use of digoxin in patients with HFrEF and chronic AF and poor control of ventricular rate and/or persistent symptoms despite optimally tolerated β-blocker therapy, or when β-blockers cannot be used (Strong Recommendation; Low-Quality Evidence).
81. We recommend that restoration and maintenance of sinus rhythm in chronic HF not be performed routinely, but individualized on the basis of patient characteristics and clinical status (Strong Recommendation; High-Quality Evidence).
82. We suggest catheter ablation of AF be considered as a therapeutic strategy to achieve and maintain sinus rhythm if rhythm control is indicated and antiarrhythmic therapy has failed or the patient is unable to tolerate antiarrhythmic therapy (Weak Recommendation; Low-Quality Evidence).
83. We recommend oral anticoagulation for AF in patients with HF unless contraindicated, as per current CCS AF guidelines,[269] and not to coadminister antiplatelet agents unless the latter are strongly indicated for other reasons (Strong Recommendation; High-Quality Evidence).
84. We suggest that non-vitamin K antagonist oral anticoagulants should be the agent of choice for stroke prophylaxis in patients with HF and nonvalvular AF, and that the treatment dose be guided by patient specific characteristics including age, weight, and renal function (Weak Recommendation; Moderate-Quality Evidence).
85. We suggest the application of evidence-based therapies for HFrEF, per CCS HF guidelines, for primary prevention of AF (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendations are on the basis of an understanding that the management of patients with HF with AF should be individualized with respect to the need to identify precipitating factors, to assess the risk of therapy such as the development of bradycardia and proarrhythmia with antiarrhythmic agents, and the bleeding risk of systemic anticoagulation.
These recommendations place a high value on the understanding that the use of cardiac glycosides in patients with chronic HF and AF remains controversial with conflicting results from meta-analyses. Digoxin can cause atrial and ventricular arrhythmias particularly in the presence of hypokalemia. Not all glycosides and not all preparations have been studied in terms of efficacy and safety.
These recommendations are consistent with the current CCS AF guidelines.[269]
In patients with HF with AF, for whom a rate control strategy is used, the heart rate treatment target remains unclear. Retrospective analyses of large RCTs suggest that rates > 110-115 bpm might be associated with worse outcomes.[269]
Practical Tip
In patients who are symptomatic from AF or whose symptoms of HF are believed to have substantial contribution from arrhythmia, consideration can be given for rhythm control.
Nondihydropyridine CCBs (eg, verapamil and diltiazem) should not be used to control heart rate in patients with HFrEF because they can depress cardiac function and worsen HF.
Dronedarone should not be used in patients with an EF < 35% and/or with recent decompensated HF because of increased risk of mortality. Agents such as sotalol, flecainide, and propafenone should also be avoided.
β-Blockers and nondihydropyridine CCBs should not be routinely combined as part of a rate control strategy in patients with HF because this might be associated with high-degree AV block.
In acutely decompensated patients with AF and HFrEF, digoxin is the first choice for heart rate control and β-blockers may be used in addition when the patient has clinically stabilized; long-term digoxin use is not often required.
Among patients with HF who receive digoxin for rate control, trough serum digoxin levels should not exceed 1.0 ng/mL.
7.2.2 CAD and revascularization
Nearly 60% of patients with chronic HF suffer from CAD, and approximately 15% of AHF cases occur in the setting of an ACS.[278],[279] Despite the coexistence of CAD and HF, few clinical trials have been performed that can inform optimal care for patients with these conditions. Several areas exist in which high-grade evidence is lacking, such as coronary revascularization in the setting of HFpEF. Although ample evidence exists to support PCI in patients with HF due to ACS,[280] there is limited evidence to support its use in the setting of chronic HF to reduce adverse clinical outcomes.[281] A detailed discussion of different imaging modalities, an approach to the diagnosis of CAD, and the perioperative management and the approach to revascularization are covered in sections 7.2.2.1-7.2.2.5 of the Supplementary Material, and Figures 6 and 7.
Recommendation
86. We recommend that noninvasive imaging for patients with HF be considered to determine the presence or absence of CAD (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation places value on identification of CAD as the cause of HF, which might have prognostic implications, and require treatments aimed toward secondary vascular prevention.
87. We recommend that coronary angiography be:
i. Performed in patients with HF with ischemic symptoms and who are likely to be good candidates for revascularization (Strong Recommendation; Moderate-Quality Evidence);
ii. Considered in patients with systolic HF, LVEF < 35%, at risk of CAD, irrespective of angina, who might be good candidates for revascularization (Strong Recommendation; Low-Quality Evidence);
iii. Considered in patients with systolic HF and in whom noninvasive coronary perfusion testing yields features consistent with high risk (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendations place value on the need for coronary angiography to identify CAD amenable to revascularization. Available evidence suggests that coronary revascularization might provide quality of life and prognostic benefits to patients with HF and noninvasive imaging delineating high risk. In particular, patients with systolic HF because of ischemic heart disease might derive clinical benefit from coronary revascularization even in the absence of angina or reversible ischemia.
Practical Tip
Several noninvasive methods for detection of CAD are in widespread use, including dobutamine stress echo, perfusion CMR, cardiac positron emission tomography testing, cardiac CT, and nuclear stress imaging. Local factors (availability, price, expertise, practice patterns) will determine the optimal strategy for imaging (Fig. 6).[277]
Noninvasive imaging modalities might provide critical information such as the amount and degree of ischemic or hibernating myocardium, and might be used to determine the likelihood of regional and global improvement in LV systolic function after revascularization.
Patients with HF and reduced LVEF are more likely to experience significant improvement in LVEF after successful coronary revascularization if they have:
- Reversible ischemia or a large segment of viable myocardium (> 30% of the left ventricle) in nuclear stress testing/viability study;
- Reversible ischemia or > 7% hibernating myocardium on positron emission tomography scanning;
- Reversible ischemia or > 20% of the left ventricle shown as viable using dobutamine stress echo;
- Less than 50% wall thickness scarring shown by LGE on CMR imaging.
7.2.2.4 Disease management, referral, and perioperative
Recommendation
88. We recommend that the decision to refer patients with HF and ischemic heart disease for coronary revascularization should be made on an individual basis and in consideration of all cardiac and noncardiac factors that affect procedural candidacy (Strong Recommendation; Low-Quality Evidence).
89. We recommend that efforts be made to optimize medical status before coronary revascularization, including optimizing intravascular volume (Strong Recommendation; Low-Quality Evidence).
90. We recommend that performance of coronary revascularization procedures in patients with chronic HF and reduced LVEF be undertaken with a medical surgical team approach with experience and expertise in high-risk interventions (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation reflects the preference that high-risk revascularization is best preformed in higher volume centres with significant experience, and known, published outcomes.
Practical Tip
anced HF therapies, by an appropriate team, should be performed before the revascularization procedure in any patient with advanced HF.
7.2.2.5 Surgical revascularization for patients with CAD and HF
The approach to the decision of coronary revascularization in patients with HF is illustrated in Figure 7. CABG surgery is indicated in adult patients with symptoms of angina, a history of HF in association with LV dysfunction (LVEF < 35%), graftable coronary arteries, and who have an otherwise good life expectancy. This recommendation is on the basis of historical data from earlier landmark clinical trials comparing medical and surgical therapy, which identified a survival benefit with CABG in patients with triple vessel CAD along with ventricular dysfunction.[283]–[286] Further details of the trials in this area are available in section 7.2.2.5 of the Supplementary Material.
The Surgical Treatment for Ischemic Heart Failure (STICH) trial sought to address 2 hypotheses: (1) does CABG improve survival in combination with optimal medical therapy for patients with HF and CAD (LVEF < 35%) who are acceptable candidates for cardiac surgery; and (2) does the additional use of SVR of an akinetic/dyskinetic anterior wall provide better outcomes than isolated CABG for eligible individuals.[287] This study evaluated patients with ischemic cardiomyopathy with or without HF symptoms and randomized them into 3 groups, namely, optimal medical therapy and CABG alone, CABG with the SVR procedure, or neither procedure. Details pertaining to the 2 hypotheses are available in the Supplementary Material.
A major concern regarding surgical revascularization in patients with LV dysfunction is a greater rate of operative mortality. A meta-analysis of 26 observational studies (3621 patients) with a preoperative LVEF < 35% showed an operative mortality of 5.4%.[288] The 2 risk calculators for surgical mortality have been recently updated: EuroSCORE II (http://www.euroscore.org/calc.html) and the (Society of Thoracic Surgeons) STS score (http://riskcalc.sts.org/ STSWebRiskCalc273/de.aspx).
Recommendation
91. We recommend consideration of coronary artery bypass surgery for patients with chronic ischemic cardiomyopathy, LVEF < 35%, graftable coronary arteries, and who are otherwise suitable candidates for surgery, irrespective of the presence of angina and HF symptoms to improve mortality, repeat hospitalization rates, and quality of life (Strong Recommendation; Moderate-Quality Evidence).
92. We suggest consideration of PCI for patients with HF and limiting symptoms of cardiac ischemia, and for whom CABG surgery is not considered appropriate (Weak Recommendation; Low-Quality Evidence).
93. We recommend against routine performance of surgical ventricular restoration for patients with HF (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendations are on the basis of data from RCTs on CABG and surgical ventricular restoration in patients with reduced systolic function and CAD, regardless of the results of viability imaging. The recommendation on PCI is on the basis of clinical need rather than RCT trial data.
Practical Tip
In the setting of HF, angina and single territory CAD, PCI might be the treatment of choice. However, PCI has not been shown to improve outcomes for patients with chronic stable HF, irrespective of underlying anatomy.
In contrast to the chronic stable patient with HF, urgent directed culprit vessel angioplasty continues to be the revascularization modality of choice for patients with ACS complicated by HF.
In highly selected cases, patients with advanced HF symptoms in association with large areas of dyskinetic and nonviable myocardium might experience clinical improvement with surgical ventricular reconstruction (SVR) or similar type procedures, when performed by experienced surgeons.
Although mitral valve repair or replacement are both considered acceptable strategies for treatment of severe mitral regurgitation (MR), it should be noted that the additional use of mitral repair has not been shown to improve survival despite technical success. This is also the case for catheter-based treatment of MR.
The Canadian Association of Cardiac Rehabilitation and CCS joint position statement includes routine cardiac rehabilitation for patients with HF who successfully complete CABG surgery.[282]
Recommendation
94. We recommend that after successful cardiac surgery, all patients be referred to a local cardiac rehabilitation program (Strong Recommendation; High-Quality Evidence).
Values and Preferences
These recommendations reflect our support of and conformity with preexisting rehabilitation guidelines statements.
7.2.3 Right heart failure
RHF is defined as the clinical syndrome in which the right ventricle function is impaired secondary to any structural or functional cardiac disorders leading to inadequate blood flow through the pulmonary circulation at a normal central venous pressure.[289] The most common reason for RHF is left-sided HF but occasionally RHF might occur as pure right-sided HF (Table 19).
In HF, RV dysfunction is a strong predictor of mortality.[290],[291] In a study of 377 patients with chronic HF who underwent right heart catheterization, 75% of patients had reduced RV function which was an independent risk of death.[292] In another study of 250 consecutive patients with dilated cardiomyopathy it was shown that reduced RV EF < 45% using magnetic resonance imaging (MRI) was an independent predictor of transplantation-free survival and worse prognosis.[293] The underlying pathophysiology of RHF might include venous congestion, RV enlargement, increased pulmonary artery pressure (PAP) and tricuspid or pulmonary valve abnormalities.
The clinical presentation of RHF is variable but typically involves fluid retention (ascites, peripheral edema), decreased systolic reserve or low cardiac output (fatigue, exercise intolerance), atrial or ventricular arrhythmias, and hypotension. Gastrointestinal symptoms like anorexia, bloating, nausea, and constipation are very common in patients with advanced RV failure. There are several medical conditions that mimic or coexist with RHF including liver cirrhosis, nephrotic syndrome, and renal failure with volume overload.
Of all physical signs of RHF, an abnormal jugular venous pressure is almost always present. In more advanced cases pitting edema, ascites, and liver enlargement are present. In the absence of elevated venous pressure, peripheral edema and ascites are unlikely to be due to RHF.
All patients with suspected RHF should undergo transthoracic echocardiography. CMR imaging has become the test of choice for noninvasive assessment of RV size, function, viability, potential etiology, and mass.[294] In selected patients with RHF, right heart catheterization should be considered to help determine etiology of RHF and provide PAP, PCWP, and PVR. PH is defined by a mean PAP of ≥ 25 mm Hg and increased PVR of > 3 Wood units with a normal PCWP < 15 mm Hg.[295]
There are very few RCTs that addressed the management of isolated RHF, recognizing that the most common cause of RHF is left heart disease. Generally, diuretics are the mainstay of therapy. Because patients with RHF might have normal or even low LV filling pressures, cautious use of diuretics is the key, because excessive diuresis can result in prerenal azotemia, hypotension, and exacerbation of arrhythmias. As such, it is not uncommon to see combination diuretic therapy to avoid excessive potassium loss or alkalosis.
Studies designed to see if treatment for LHF also ameliorates RHF failed to show benefit because these studies were underpowered or mechanisms of injury and repair might differ.[296]–[299] Patients with RHF secondary to congenital heart disease or secondary to PH should be referred early to specialized clinics for investigations and management.[300],[301]
Cor pulmonale term is used in cases of RHF associated with pulmonary hypertension as a result of lung disease. Determination of the etiological factor is of utmost importance because several therapies specific to the underlying cause have been developed (Table 20). For these reasons, patients with HF and PH (without LV failure) should be referred to centres with experience and expertise in the management of this disorder. In particular, patients with congenital heart disease might present with RHF due to a wide variety of specific anomalies or surgical residua. When identified, these patients should be referred to an adult congenital heart disease centre.[301]
The diagnosis of cor pulmonale should be considered in all patients with lung disease and symptoms and/or signs of RHF. The tests used for the diagnosis of cor pulmonale include chest x-ray, ECG, echocardiogram (ECHO), CT scan, ventilation/perfusion lung scanning, MRI, pulmonary function test, and right heart catheterization. In some cases lung biopsy might be required to determine the underlying cause for cor pulmonale. The treatment of PH and cor pulmonale is determined by the etiology and specific management of these patients is addressed in disease-specific guidelines.[302],[303] Patients with PH and cor pulmonale should be referred to the centres with appropriate expertise for the confirmation of diagnosis, vasoreactivity testing, and institution of appropriate treatment.
7.2.3.1 Arrhythmogenic right ventricular cardiomyopathy
ARVC is a genetic form of cardiomyopathy characterized by fatty/fibrofatty infiltration of the myocardium affecting mostly the right ventricle but occasionally the left ventricle too.[304] ARVC is an autosomal dominant inherited disease with variable penetrance and expression. Prevalence of ARVC is estimated to be 1:1000-1:5000[305],[306] affecting men more frequently than women with a ratio of 1:3.[307]
ARVC should be suspected in a patient with unexplained RV dysfunction, dilation, or RHF, a history of ventricular tachyarrhythmia (particularly of LBBB morphology) or syncope, characteristic ECG changes (eg, epsilon waves), a family history suggestive of syncope or sudden death, and in young people or athletes with a history of syncope or cardiac arrest during exercise or sports activities.
In 2010, the 1994 European Society of Cardiology/International Society and Federation of Cardiology joint task force criteria for ARVC were revised by introducing quantitative measures into the criteria.[308] Current task force criteria for ARVC diagnosis include diagnostic criteria on the basis of 6 categories: typical ECG findings (eg, epsilon waves), ventricular arrhythmias (left bundle block morphology), morphological and functional changes in the right ventricle, histopathology, family history, and genetic findings. On the basis of these criteria, 3 levels for the ARVC diagnoses were established. Major and minor criteria for ARVC are listed in Table 21.[308]
Echocardiography and CMR imaging are the most commonly used tests for the diagnosis of ARVC.[309] Strain echocardiography is one of the newest modalities and it appears to be very effective in the assessment of RV function.[310] CMR imaging if available is a preferred test to echocardiography for ARVC.[311] It can provide very accurate information on LV and RV function and the presence of intramyocardial fat and fibrosis via LGE. In some patients myocardial biopsy might be useful but diagnostic sensitivity is very low because of the patchy distribution of the disease.
Genetic testing can be useful in patients with a diagnosis of ARVC however, an existing known gene mutation is present in only approximately 50% of cases. Therefore, negative gene testing does not rule out ARVC.
The primary goal of treatment of ARVC is to identify high-risk patients to reduce the risk of sudden arrhythmic death. The secondary goal of treatment is to manage symptoms of ventricular arrhythmias and RHF. To date, there have been no RCTs to determine the efficacy of pharmacological and device therapies on the prevention of sudden death. Patients with ARVC and RV or LV failure should be treated with standard medical therapy. Antiarrhythmic medications have been used frequently to decrease frequency of ICD discharges.[312] β-blockers have shown effectiveness in reducing adrenergically stimulated arrhythmias.[313]
ARVC patients at high risk for sudden death on the basis of a clinical profile with one or more risk factors for sudden death should be considered for ICD as a primary prevention.[314] For secondary prevention of sudden death in the ARVC population, ICDs are indicated.[315] Cardiac transplantation is an option for eligible patients with advanced ARVC and intractable HF or ventricular tachyarrhythmia.
Exercise has been linked to the increased risk of ventricular tachycardia secondary to the elevated levels of catecholamines during physical activity in the setting of ARVC.[316] Patients with suspected or confirmed ARVC should avoid vigorous physical activity like competitive sports, or regular activities associated with symptoms.[317],[318]
All patients with ARVC should be referred to experienced centres with electrophysiology services and genetic counselling.
7.2.3.2 Constrictive pericarditis
Constrictive pericarditis is an uncommon disease of pericardium resulting from chronic inflammation and fibrosis leading to impaired diastolic filling of the ventricles with or without reduced systolic function. In developing countries, the most common reason for the constrictive pericarditis is tuberculosis.[319]
Constrictive pericarditis should be suspected in patients with unexplained RHF in whom there is a history of pericardial disease or predisposing pericardial injury. The most commonly reported symptoms are peripheral edema and exertional dyspnea. Almost all patients have abnormal jugular venous pressure. It is elevated with very prominent, deep y-descent and frequently there is an increase in the jugular venous pressure with inspiration (Kussmaul sign). Pulsus paradoxus is frequently present as are an enlarged liver and ascites.
The diagnosis of constrictive pericarditis is frequently delayed because clinical presentation is often atypical and might mimic other causes of the RHF.[320] Chest x-ray is very helpful in diagnosis of constrictive pericarditis because in 27% of cases, calcification can be seen in the pericardium.[321] In cases in which diagnosis of constrictive pericarditis is suspected, a special “constriction protocol” should be requested when ordering an ECHO.[322] It should include a focus on the motion of the ventricular septum, variation in the mitral inflow velocity, variation in the hepatic vein profile, and tissue Doppler assessment of mitral annular velocities with simultaneous recording of respiration and tissue strain.[323]–[325] A CT scan is helpful in the assessment of pericardial thickness and calcifications. CMR imaging can provide anatomical details, hemodynamic information, and an assessment of pericardial inflammation. When noninvasive evaluation is indeterminate, cardiac catheterization with hemodynamic assessment is the test of choice.[326]
Management includes treatment to relieve symptoms of RHF, control secondary arrhythmia, and provide timely surgical consultation for pericardiectomy. Transient constrictive pericarditis post cardiac surgery can resolve spontaneously or after a short course of anti-inflammatory medications or corticosteroids.[327]
All patients with constrictive pericarditis should be referred to experienced centres with advanced cardiac imaging, catheterization, and surgical availability for assessment and treatment. All symptomatic patients with chronic constrictive pericarditis should be considered for pericardiotomy. Current surgical mortality rates average 6%-12%,[328],[329] but can be elevated further if there is coexisting myocardial damage, extensive pericardial calcification (‘outer porcelain heart’), or previous mediastinal radiation.
Recommendation
95. We recommend RHF should be considered in patients with unexplained symptoms of exercise intolerance or hypotension in combination with evidence of elevated jugular venous pressure, peripheral edema, hepatomegaly, or any combination of these findings. Echocardiography should be performed to assess cardiac structure and function, and inferior vena cava dispensability. In cases of refractory RHF, or when the diagnosis is not clear, hemodynamic assessment with complete right heart catheterization should be considered (Strong Recommendation; Low-Quality Evidence).
96. We recommend that patients with RHF secondary to or in association with left HF (LHF) should be managed as per LHF guidelines (Strong Recommendation; High-Quality Evidence).
97. We recommend judicious diuretic therapy for patients with symptomatic RHF, with a goal of euvolemia if feasible and tolerated (Strong Recommendation; Low-Quality Evidence).
Practical Tip
Cor pulmonale is RHF caused by pulmonary arterial hypertension (PH), which is usually a consequence of lung disease. Cor pulmonale should be suspected in patients with PH or lung disease who also have signs and/or symptoms of RHF.
Recommendation
98. We recommend patients with PH undergo evaluation in centres with experience and expertise in the management of this disorder (Strong Recommendation; Low-Quality Evidence).
99. We recommend that right heart catheterization be considered in selected patients with right-sided HF to determine the true pulmonary artery systolic pressure, pulmonary vascular resistance (PVR), transpulmonary gradient, and pulmonary capillary wedge pressure (PCWP), and to exclude left-sided HF as the underlying cause (Strong Recommendation; Low-Quality Evidence).
100. We recommend cardiologist referral for patients with any right-sided obstructive cardiac lesion and moderate or severe right-sided regurgitant lesion for assessment of etiology, associated diseases, and treatment plan (Strong Recommendation; Low-Quality Evidence).
101. We recommend that symptomatic patients with severe right-sided obstructive or severe regurgitant lesions be evaluated and considered for surgical or percutaneous intervention at a centre with expertise and experience in the management of these conditions (Strong Recommendation; Low-Quality Evidence).
102. We recommend that patients with severe (peak gradient > 80 mm Hg) or symptomatic moderate (peak gradient 50-79 mm Hg) pulmonary valvular stenosis should be referred or considered for balloon valvuloplasty or surgical intervention (Strong Recommendation; Low-Quality Evidence).
103. We recommend bioprosthetic rather than metallic prosthesis for replacement of right-sided valvular lesions (Strong Recommendation; Low-Quality Evidence).
104. We recommend diagnosis of arrhythmogenic RV cardiomyopathy (ARVC) be made according to the European Society of Cardiology/International Society and Federation of Cardiology criteria (revised in 2010) (https://www.escardio.org/Working-groups/ Working-Group-on-Myocardial-and-PericardialDiseases/Publications/Paper-of-the-Month/Diagnosisof-arrhythmogenic-right-ventricular-cardiomyopathydysplasia) to establish a diagnosis (Strong Recommendation; Low-Quality Evidence).
105. We recommend individuals with ARVC avoid strenuous or high-intensity sports activities (Strong Recommendation; Moderate-Quality Evidence).
106. We recommend an ICD be offered to all eligible patients with ARVC who have had a cardiac arrest or a history of sustained ventricular tachycardia (Strong Recommendation; Low-Quality Evidence).
107. We recommend an ICD be considered for the prevention of SCD in eligible patients with ARVC in whom the risk of SCD is judged to be high (Strong Recommendation; Low-Quality Evidence).
108. We recommend all patients with ARVC be referred to a centre with experience and expertise in the management of this condition (Strong Recommendation; Low-Quality Evidence).
109. We recommend genetic counselling be considered for families with ARVC for the purpose of screening and/or genetic testing (Strong Recommendation; Low-Quality Evidence).
110. We recommend CT scan or CMR imaging be performed in all patients with suspected constrictive pericarditis to assess for pericardial thickening (Strong Recommendation; Low-Quality Evidence).
111. We recommend that echocardiography with Doppler assessment of ventricular filling, as well as a right- and left-sided (simultaneous) cardiac catheterization (with manoeuvres if necessary) be performed in all cases of constrictive pericarditis to confirm the presence of a constrictive physiology (Strong Recommendation; Low-Quality Evidence).
112. We recommend surgical referral for pericardiectomy be considered for patients with constrictive pericarditis and persistent advanced symptoms despite medical therapy (Strong Recommendation; Moderate-Quality Evidence).
113. We recommend that patients with symptomatic constrictive pericarditis be offered referral to a centre with expertise in the management of this condition (Strong Recommendation; Low-Quality Evidence).
Practical Tip
Atrial septal defect might be difficult to diagnose and should be suspected in the setting of unexplained RHF or RV enlargement. Bubble study or transesophageal echocardiography might be required for diagnosis.
Patients with RHF might not have increased left atrial filling pressures and might be more sensitive to change in reduction of cardiac preload and renal dysfunction. This might manifest as light-headedness or elevation of serum creatinine level. Careful monitoring of volume status is necessary.
Patients with RHF might require increased doses of diuretics, which might lead to increased likelihood of hypokalemia. Judicious use of potassium-sparing diuretics might be useful in the maintenance of potassium homeostasis.
Carefully selected patients with advanced HF and severe pulmonary hypertension during optimal therapy might be considered for therapy with sildenafil for improvement of symptoms and exercise tolerance.
Ventilation/perfusion lung scanning should be used as a screening test for chronic thromboembolic pulmonary hypertension but CT pulmonary angiography or conventional pulmonary angiography will be required for the confirmation of chronic thromboembolic pulmonary hypertension diagnosis.
Pulmonary function testing with diffusion of carbon monoxide should be performed to determine underlying obstructive or interstitial lung disease.
Lung biopsy might be considered for diagnosis in cases in which the diagnosis is in doubt and will refine treatment.
Evaluation for lung and heart-lung transplantation should be considered for end-stage cor pulmonale.
Patients with trivial (mean gradient < 25 mm Hg) or mild (gradient < 50 mm Hg) pulmonary stenosis require no intervention or exercise limitation, but should have periodic follow-up (approximately every 5 years).
Patients with right-sided valvular stenosis might have underlying carcinoid syndrome or ingestion of appetite suppressants.
ARVC should be suspected in individuals with unexplained dilation or dysfunction of the right ventricle in whom there is a history of ventricular arrhythmia, syncope, or HF, or in whom characteristic ECG changes or a positive family history of ARVC is noted.
Up to 40% of patients with ARVC might have a normal ECG on initial presentation, although almost all patients will develop pathological ECG changes within 6 years.
Interpretation of CMR imaging for ARVC should be performed at experienced centres. An abnormal scan in isolation is not diagnostic for ARVC.
Endomyocardial biopsy (EMB) of the RV free wall for ARVC should be performed with extreme caution and at an experienced centre because of the high risk of myocardial perforation and cardiac tamponade.
Antiarrhythmic drugs or catheter ablation should not be used in the place of ICD therapy for patients with ARVC, but might be considered in patients who refuse or who are not candidates for device therapy.
Transthoracic echocardiography is insensitive for detecting pericardial thickening but is a useful first test for examining constrictive physiology; transesophageal echocardiography might further improve the sensitivity over the transthoracic approach.
When extensive calcification of the pericardium is present, CT imaging might be more effective than CMR imaging for measuring pericardial thickness.
Provocation testing in the cardiac catheterization laboratory, such as rapid volume loading (eg, I.V. infusion of 1 L of normal saline over 6-8 minutes) and simultaneous LV and RV measurement during respiration, might unmask hemodynamic signs of constriction in patients with early or occult forms of constrictive pericarditis.
The diagnosis of pericardial constriction might be difficult and is made on clinical grounds with supporting information from diagnostic testing. Despite extensive workup, information from EMB or even at open thoracotomy might be required to assist in the diagnosis.
7.3 Noncardiovascular comorbidities
Although treatments that improve survival, exercise capacity, and reduce hospitalizations have been established for HFrEF, the increased complexity of patients and their comorbidities often confound treatment. The latter issues have proven even more challenging for the HFpEF population. Comorbid conditions become risk factors for future deterioration and might contribute to clinical deterioration, complicate management, and are often associated with poor prognosis.
7.3.1 Anemia and iron deficiency
Anemia is often defined according to knowledge of normal, age- and sex-specific values of Hb, or hematocrit. The World Health Organization (WHO) defines anemia as a Hb level < 130 g/dL for men and 120 g/dL for women[330]; other definitions also exist.
Anemia has a prevalence ranging from 10% to 68%[331],[332]; a wide range attributable to the various definitions used and populations studied. Factors associated with anemia in chronic HF include older age, diabetes, chronic kidney disease (CKD), more advanced HF, recent HF hospitalizations, signs of HF, higher levels of neurohormones and inflammatory markers, exercise intolerance, and reduced quality of life.[333]–[337]
The prevalence of anemia is similar whether EF is reduced or preserved.[334],[338] Although anemia in HF was once thought to be almost solely attributable to CKD; anemia and CKD are now both established independent predictors of mortality and hospitalizations for HF.[339],[340] Even small reductions in Hb levels are associated with worse outcomes and even mild anemia is associated with worsening of symptoms, increased NYHA class, and impairment in functional capacity and quality of life. Anemia is associated with higher costs of hospitalization for patients with HF.[341],[342] A decline in Hb over time is also associated with mortality and morbidity.[335] Therefore, there has been continued interest for more than a decade in finding appropriate treatments for anemia in patients with HF, although the underlying pathophysiology is complex and remains only partly understood. The main mechanisms include CKD, inflammation, hemodilution, absolute or relative iron deficiency (ID), rarer nutritional deficiencies (vitamin B12, folic acid, thiamine), gastrointestinal blood loss, and a few therapeutic agents with a generally low effect on Hb levels (eg, ACEis) (Table 22). In HFrEF, increased myocardial remodelling, inflammation, and volume overload have been described as the hallmarks of patients with anemia and HF.[333]
In the CHARM studies, the effect of anemia on the primary outcome of cardiovascular death or HF hospitalization was slightly less in HFpEF than in HFrEF.[334] However, observational and population-based studies suggest that the effect of anemia on prognosis appears similar for HFpEF and HFrEF.[343],[344]
As for any other group of patients, reversible causes of anemia should be sought and treated. Beyond this first step, treatment options for patients with HF include evaluation of the contribution of volume overload, of concomitant medications (eg, antiplatelet agents, anticoagulants, and especially their combination); identification of ID and treatment with oral or intravenous (I.V.) iron supplements; and optimization of HF therapy.
Recommendation
114. We recommend that anemia be investigated and reversible causes treated (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
Multiple clinical trials and population studies have shown the prognostic effect of anemia. Reversible causes of anemia are common and should be treated.
Practical Tip
Anemia in patients with HFrEF is associated with more advanced HF, active ventricular remodelling processes, inflammation, renal dysfunction, and volume overload. Optimization of therapies directed at HF pathophysiology and volume control should therefore improve anemia.
7.3.1.1 Iron deficiency
Iron is necessary for optimal hematopoiesis but also plays a central role in oxygen transport (Hb), storage (myoglobin), cardiac and skeletal muscle metabolism; synthesis, and degradation of proteins, lipids, ribonucleic acids, and for mitochondrial function. ID, either absolute or functional, has emerged as another independent predictor of outcomes[332],[345] and a major contributor to exercise intolerance in HF, even in the absence of anemia.[346] ID might be detected before anemia appears, thus providing an earlier opportunity in view of improving outcomes. In a cohort of 1506 patients with chronic HF, ID, defined as a ferritin level < 100 mg/L or ferritin 100-299 mg/L if the transferrin saturation was < 20%, had a prevalence of 50%.[345] It is estimated that 60% of patients with HF with anemia and 40% of those without anemia have ID.[347] This definition might underestimate the prevalence of ID.
Functional ID is seen when there is a deficit in the mobilization of iron from tissues while iron stores are normal, which is frequent in chronic diseases with inflammation.[348],[349] Hepcidin, soluble transferrin receptor and reticulocyte Hb have been proposed as more sensitive indices to evaluate ID.[347],[350],[351]
A meta-analysis by Avni and colleagues[352] had reported improvements in quality of life, 6-minute walk distance, and all-cause hospitalization with iron replacement therapy. Qian and colleagues[353] reported on the effects of I.V. iron therapy on clinical outcomes in HF, in a second meta-analysis, including a total of 907 patients from 5 clinical trials. There were no increases in adverse events with I.V. iron therapy, using iron sucrose or ferric carboxymaltose (FCM) in these studies. Most patients included in that meta-analysis came from 2 larger trials, Ferric Carboxymaltose Evaluation on Performance in Patients With Iron Deficiency In Combination With Chronic Heart Failure (CONFIRM-HF) (n = 301)[354] and Ferric Carboxymaltose Assessment in Patients With Iron Deficiency and Chronic Heart Failure (FAIR HF) (n = 459).[355] The CONFIRM-HF trial[354] included 301 patients (251 completed the trial) with moderate HF symptoms (NYHA class II-III), LVEF ≤ 45%, elevated BNP or NT-proBNP, and ID. I.V. iron was given as a FCM solution equivalent to 500 or 1000 mg of iron. At week 24, the primary end point of change in the 6-minute walk distance improved more in the FCM group (difference, 33 ± 11 m; P = 0.002). The benefit was maintained up to 52 weeks. Fatigue, NYHA class, and quality of life scores also improved on through week 52, and FCM was associated with a reduction in the risk of hospitalization due to worsening HF. The Effect of Ferric Carboxymaltose on Exercise Capacity in Patients With Iron Deficiency and Chronic Heart Failure (EFFECT-HF) study (n = 173) also recently reported benefits of I.V. FCM on peak VO2 compared with standard of care in patients with HF, EF ≤ 45%, and ID.[353],[356],[357]
In patients with HF with anemia and ID in whom iron repletion is being contemplated, iron supplementation should be considered to improve functional capacity.[358] Oral iron has no known efficacy in this context, and therefore I.V. iron should be given considering the recently presented results from the Iron Repletion Effects on Oxygen Uptake in Heart Failure (IRONOUT HF) study. In that study (n = 225), high-dose oral iron minimally repleted iron stores and did not improve peak VO2 in patients with ID and HFrEF.[359] It is essential that the causes of absolute ID (such as iron loss) have also been investigated and treated by other means when possible (eg, endoscopy for gastric ulcer, colon cancer, etc). In such cases, concomitant I.V. iron therapy can reduce the time needed to correct anemia, as well as the need for transfusions.
Further evidence is warranted regarding the effect of I.V. iron repletion on major cardiovascular events (namely death), especially for nonanemic patients with HF and those with HFpEF. Cost-effectiveness also requires further validation with various I.V. iron formulations.
Recommendation
115. We recommend that I.V. iron therapy be considered for patients with HFrEF and ID, in view of improving exercise tolerance, quality of life, and reducing HF hospitalizations (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
The CONFIRM-HF trial, 3 meta-analyses, and the recent EFFECT-HF trial have improved the quality of evidence regarding benefits of I.V. iron therapy on the previously discussed outcome measures but there is yet no evidence regarding benefits on mortality. Because of the rapid rate of iron repletion using the I.V. route and the available evidence, this treatment should be considered rather than oral iron repletion. Ongoing hospitalization can provide a good opportunity to facilitate I.V. iron administration.
Practical Tip
ID can be difficult to diagnose in patients with HF and diagnosis should ideally be done in a clinically stable state. The most widely accepted definition is a serum ferritin < 100 mg/L or ferritin between 100 and 299 mg/L and transferrin saturation < 20%. New biomarkers, such as soluble transferrin receptor, hepcidin, and reticulocyte Hb might improve the sensitivity and specificity for the diagnosis of ID; but their clinical utility has yet to be shown.
7.3.1.2 Erythropoiesis-stimulating agents
ESAs have been studied as a potentially promising class of agents to increase Hb in HF, considering not only CKD but multiple proposed pleiotropic properties of such agents. The 2 largest trials on ESAs in HF were the Study of Anemia in Heart Failure Trial (STAMINA-HeFT)[360] and the Reduction of Events with Darbepoetin Alfa in Heart Failure (RED-HF) trial.[361] These 2 trials, and a meta-analysis[362] failed to show benefits on mortality, cardiovascular events, and hospitalizations. In RED-HF, a significant increase in thromboembolic events was reported in patients with Hb levels > 130 g/dL.[361] On the basis of the results of those studies, it is unlikely that another morbidity or mortality study will be undertaken with results that will support the use of ESAs in HF.
Although ESA administration is common practice in advanced CKD, the debate continues on target Hb and the effect on quality of life, which is likely higher for those with lower Hb levels.[363] Patients with advanced CKD (eGFR < 30 mL/min/1.73 m2) should be managed in concert with nephrologists, especially when hemodialysis is contemplated. In those cases, as well as in some hemato-oncologic conditions, ESA therapy might be an option.
Recommendation
116. We recommend erythropoiesis-stimulating agents (ESAs) not be routinely used to treat anemia in HF (Strong Recommendation; High-Quality Evidence).
Values and Preferences
This recommendation against the use of ESAs for the treatment of anemia in HFrEF at large was derived from robust data from RCTs.
Practical Tip
with severe CKD or hemato-oncologic conditions might benefit from an ESA and should be referred to a specialist with expertise in such treatments, for proper initiation and follow-up.
7.3.2 Diabetes(treatment)
7.3.2.1 Glycemic control in patients with diabetes and HF
With the available evidence, an intensive glycemic control strategy cannot be recommended for all patients with diabetes. Instead, each individual should be assessed for his or her optimal glycemic target for the prevention of macrovascular events.
7.3.2.2 Pharmacological therapy for type 2 diabetes in patients with HF
Metformin. Metformin is still considered first-line pharmacological therapy for type 2 diabetes.
Recommendation
117. We suggest that metformin be considered a first-line agent for type 2 diabetes treatment (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
Metformin is the current Canadian Diabetes Association first-line treatment for type 2 diabetes.
SGLT-2 inhibitors. Ongoing trials of SGLT-2 inhibitors will inform the use of the class of agents in patients with established HF. Few patients in the EMPA-REG OUTCOME trial had HF at baseline (approximately 10%), however, patients with HF had results similar to those in the overall trial.[27] There are ongoing trials of SGLT-2 inhibitors specifically enrolling patients with HF that might inform future recommendations.
DPP-4 inhibitors. There are no high-quality HF-specific studies from which to provide guidance for patients with established HF.
GLP-1 agonists. Several small trials have tested the additional use of a GLP-1 agonist in patients with HF. None have shown any additional benefit in patients with HF, with or without diabetes.[364],[365]
Thiazolidinediones. Two such thiazolidinedione drugs (pioglitazone and rosiglitazone) have each been shown to increase the risk of HF events and should be avoided in patients with HF, as summarized in section 4.3.1. Glycemic Control in Diabetes to Prevent HF.
Recommendation
118. We recommend that thiazolidinediones should not be used in patients with HF (Strong Recommendation; High-Quality Evidence).
Values and Preferences
Pioglitazone as well as rosiglitazone have been shown in studies to increase the risk for HF.
7.3.3 Cardiorenal syndrome
Cardiac and renal dysfunction often occurs in concert with hemodynamic, neurohormonal, vascular, and hematologic consequences. Previously, renal dysfunction was thought to represent merely comorbidity in patients with advanced HF. It is increasingly recognized that cardiac and renal interaction is complex. Cardiorenal syndrome (CRS) refers to interactions in which renal dysfunction and HF interact and mutually reinforce each other.[370] Mechanistic hypotheses are discussed elsewhere.[370],[371] Elevated intra-abdominal pressure as well as central venous pressure are linked to rising serum creatinine levels. Elevated renal vein hydrostatic pressure is an important mechanism in volume-expanded patients.[372],[373]
When managing these patients the Acute Dialysis Quality Initiative (ADQI) definition of CRS should be followed (Table 23).[371],[374]
Recommendation
119. We recommend that patients with CRS should be managed by a multispecialty team that has expertise in this area (Strong Recommendation; Low-Quality Evidence).
120. We suggest that for patients with persistent volume overload despite optimal medical therapy and increases in loop diuretics, cautious additional use of a second diuretic (a thiazide/low-dose metolazone) may be considered as long as it is possible to closely monitor morning weight, renal function, and serum potassium (Weak Recommendation; Moderate-Quality Evidence).
121. We suggest that patients with CRS who develop diuretic resistance should be tried on stepped pharmacologic therapy (Weak Recommendation; Low-Quality Evidence).
Values and Preferences
These recommendations place a high value on the understanding that diuretics have not been shown to improve survival but are frequently required to relieve congestion.
Practical Tip
Serum potassium should be maintained at 4-5.5 mmol/L. Serum magnesium levels should be checked if there is persistent or resistant hypokalemia or the patient develops muscle cramps or ventricular arrhythmia, but has no additional proven benefit to test or replace magnesium in routine HF care.
A meta-analysis of observational studies confirms that patients with HF with moderate to severe renal dysfunction have more than a twofold increase in relative mortality risk.[366] The presence of HF in a hemodialysis population portends a poor prognosis with mean survival of < 36 months.[367]
Creatinine clearance calculated using Cockcroft and Gault,[368] Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), or the Modification of Diet in Renal Disease formula[369] is used to estimate the glomerular filtration rate (GFR). Standardized and validated criteria might be useful to estimate acute changes in renal function at the bedside for patients with AHF when renal injury is a possibility. The National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines should be used for the classification, and evaluation of CKD (Table 24).[369] It is important to recognize that markers used in the assessment of HF including BNP and NT-proBNP might need to be interpreted with caution in the presence of acute renal failure or end-stage renal disease.
Recommendation
122. We recommend that HF patients with stable, chronic mild-to-moderate renal insufficiency (GFR > 30) should receive standard therapy with an ACEi or ARB and an MRA (Strong Recommendation; Moderate-Quality Evidence).
Practical Tip
Because the isolated measurement of serum creatinine might not accurately reflect the degree of renal function we recommend using the eGFR when evaluating renal function. The standard definition of chronic renal insufficiency should be used when evaluating renal function.
No large, randomized, outcome trials on the use of diuretics have been conducted in patients with renal insufficiency.
Monitoring of electrolytes and creatinine in patients with CRS should be more frequent especially with acute illness, dehydration, and when increasing the doses of cardiac drugs, including diuretics.
Changes in GFR after commencing therapy are not necessarily associated with worsening outcome.
As a general rule, the serum creatinine level can rise or eGFR level can decrease by as much as 30% from baseline before it becomes necessary to stop or reduce the dose of the ACEi, ARB, or MRA.
Recommendation
123. We recommend that in all cases, potential reversible causes for declining renal function must be excluded and referral to a nephrologist should be considered (Strong Recommendation; Moderate-Quality Evidence).
124. We recommend that digoxin should be avoided in patients with acute renal injury and in patients with chronic, severe renal insufficiency (GFR < 30). In mild-to-moderate, stable renal insufficiency, digoxin should be used judiciously, at a low dose. As renal function declines, digoxin usage should be reassessed to avoid development of digoxin toxicity (Strong Recommendation; Low-Quality Evidence).
7.3.3.1 Role of hemodialysis
Hemodynamic stress, metabolic changes, and electrolyte shifts often occur in patients receiving hemodialysis and might be poorly tolerated. Dialysis should be considered in patients with HF with signs and symptoms of complications of renal failure.[375] Early consultation with a nephrologist is recommended for patients with acute kidney injury and in situations in which dialysis is being considered.
Before initiating dialysis, clinicians should be aware of the poor prognosis of patients with HF and end-stage renal disease (see section 8. Community Management of HF). When initiated, clinicians and patients might have difficulty accepting the need to discontinue or continue dialysis. Common complications associated with dialysis include dehydration and electrolyte imbalance, which might lead to angina, hypotension, or arrhythmias if left untreated. A reduction in medical therapy might be necessary for effective hemodialysis to occur, and there should be caution when reintroducing these at a later time.
Medications specific to HF should be continued for patients treated with hemodialysis when possible. An RCT of carvedilol in 114 hemodialysis patients with a low LVEF and HF symptoms showed a significant reduction in mortality or hospitalization over 2 years and improvement in NYHA and LV remodelling.[376] Cohort data suggest that ACEis are associated with a reduction in all-cause mortality.[377] A single RCT of 397 high-risk patients without HF receiving hemodialysis showed a trend toward a lower event rate with the use of fosinopril.[378] Similarly, ARBs have been tested in an openlabel trial in hemodialysis patients[379] and were not reported to reduce clinical events. One randomized trial of 332 patients with HF receiving an ACEi and hemodialysis showed a significant all-cause mortality reduction over 3 years with the ARB telmisartan but with significantly more hypotension and dropouts in the treatment arm.[380] These results do not alter previous recommendations on combination therapy with an ACEi and an ARB. Aldosterone blockade has been evaluated in 3 small cohorts for safety[381]–[383] and 1 small RCT of 16 patients with HF without significant benefits.[384] There are limited safety data and no efficacy data favouring digoxin for patients with HF receiving hemodialysis.
Recommendation
125. We recommend starting or continuing the use of ACEis/ARBs, and β-blockers in patients with HF and receiving chronic dialysis (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
The use of MRAs in hemodialysis patients with HF has been shown to reduce mortality.
7.3.3.2 Role of renal transplantation
Renal transplantation is an option for selected candidates with HF according to internationally accepted guidelines.[385] Three cohort studies have highlighted the importance of the cardiorenal interaction in patients who have undergone renal transplantation and subsequently had improvement in symptoms, LV function, and remodelling.[386],[387] Postoperative adverse cardiac events were low (< 5%) in these patient cohorts.
7.3.3.3 Role of ultrafiltration
Diuretic therapy is the mainstay for relief of volume overload for AHF.[388] However, evidence-based data are sparse and diuretics have adverse effects such as activation of the neurohormonal cascade, electrolyte depletion, and renal injury.[389],[390] Venovenous UF has been evaluated as an alternative therapy in this setting. Potential advantages of UF include greater control over the rate and volume of fluid removal, greater net loss of sodium, and less neurohormonal activation.[391]
In the multicentre randomized controlled Relief for Acutely Fluid-Overloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial the feasibility, safety, and efficacy of early UF vs usual care in the management of AHF was assessed.[392] Early UF resulted in a trend toward greater weight loss and fluid removal at 24 hours. UF was well tolerated and the median volume of ultrafiltrate removed during a single 8-hour course of UF was 3213 mL. Dyspnea and HF symptoms were improved in the UF group at 48 hours.
In the Ultrafiltration versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Congestive Heart Failure (UNLOAD) trial, early UF was compared with standard I.V. diuretic therapy in patients with AHF.[393] UF produced greater weight loss and net fluid loss over 48 hours but no difference in dyspnea scores, creatinine level, or length of stay. There was an associated decrease in HF rehospitalization at 90 days but other important clinical outcomes were not affected. The studies on UF were not powered to address major clinical outcomes, and no long-term evaluation (> 90 days) of the effect on HF or all-cause hospitalizations has been performed.
In a randomized trial Caridorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) of UF in decompensated HF with CRS, stepped pharmacologic therapy using I.V. diuretics and other medications in an organized fashion was reported to be superior to UF for the preservation of renal function, with similar weight loss. A higher percentage of patients in the UF group had a serious adverse event.[394]
The potential risks associated with UF include hypotension, bleeding, hemolysis, catheter-related complications, allergic reactions, air emboli, and worsening renal function. Currently, patients receiving UF require systemic anticoagulation, placing an additional risk of systemic bleeding. Estimates regarding the safety of UF, on the basis of the published literature, are from small RCTs and cannot be readily extrapolated to a broader population and in centres without experience and expertise in UF.
Recommendation
126. We do not recommend the routine use of ultrafiltration (UF) for the management of intractable edema in decompensated HF (Weak Recommendation; Low-Quality Evidence).
Practical Tip
Although the routine use of UF for volume and symptom control in HF is not supported by trial evidence, it could be tried in refractory cases for patients in whom stepped pharmacologic therapy has failed. All forms of UF should be avoided unless it is used, as a last resort for symptom control in a centre well versed in its use with the understanding that it could further compromise renal function and that the benefits are short-lived.
7.3.4 Sleep apnea
7.3.4.1 Sleep disordered breathing in HF
The subject of sleep apnea, generally referred as sleep disordered breathing (SDB) in patients with HF has recently been extensively reviewed.[395] There are 2 types of SDB, namely obstructive sleep apnea (OSA) and central sleep apnea (CSA), which operate through different pathophysiological mechanisms, although they can interact with each other.[396] OSA results from collapse of the pharynx. Struggling to breathe causes generation of negative intrathoracic pressure, leading to loading of the ventricles. CSA results from either a reduction in central respiratory drive or instability in feedback control of the central respiratory centre. It might be a consequence of HF, but when present, increases the risk of arrhythmias and worsen prognosis.[396]
About 40% of patients with HF have CSA and 11% have OSA.[396]–[398] A significant number patients with HF with SDB remain undiagnosed, possibly because of a lack of awareness and limited availability of sleep laboratories. Although nocturnal polysomnography in a sleep laboratory is the preferred diagnostic method,[399] home-based unattended sleep studies have been used as screening tools.[396] Patients with HF should be asked screening questions, such as on snoring and falling asleep during the day.
7.3.4.2 Treatment of SDB
There have been several RCTs of CPAP therapy in patients with HF and OSA or CSA. For OSA, small studies consistently showed that CPAP reversed obstructive apnea and improved oxygenation at night.[396] There are also small RCTs of CPAP in CSA, when applied for at least 1-3 months, reduced the apnea-hypopnea index and improved EF and functional class.[396] One study reported a reduction in combined mortality and heart transplantation rate in HF with CSA, but not in patients without CSA,[400] which in part prompted the conduct of the Canadian Continuous Positive Airway Pressure for Patients With Central Sleep Apnea and Heart Failure (CANPAP) trial.[401] This study was terminated early because of a lower than expected event rate. Despite a reported 50% reduction in the apneahypopnea index and 6-minute walking distance, the transplantation-free survival and rates of HF-related hospitalizations were identical in both groups. Thus, data to date suggest that although CPAP alleviated CSA and improved cardiac function, its effectiveness in improving clinical outcomes remains unclear.
The adaptive servo-ventilator has been designed for the treatment of CSA and provides a baseline degree of ventilatory support, in which the patient’s ventilation is servocontrolled to maintain the ventilation at 90% of the long-term average.[402] Short-term RCTs have shown abolition of CSA but an inconsistent effect on EF.[395] In the recently published Adaptive Servo Ventilation in Patients With Heart Failure (SERVE-HF) trial,[403] 1325 patients with an LVEF ≤ 45%, apnea-hypopnea index ≥ 15 events per hour, and a predominance of central events were randomized to receive medical treatment with adaptive servoventilation or medical treatment alone. The primary end point of death, cardiovascular interventions, and HF hospitalization, was not altered. However, all-cause mortality and cardiovascular mortality were significantly higher in the adaptive servo-ventilation group than in the control group. The ongoing Effect of Adaptive Servo Ventilation on Survival and Hospital Admissions in Heart Failure (ADVENT-HF) trial (NCT01128816) might provide more insight into the role of servo-ventilation in HF.
Recommendation
127. We suggest that patients with HFrEF and CSA not be treated with adaptive servo-ventilator treatment (Weak Recommendation; Moderate-Quality Evidence).
128. We suggest that physicians treating patients with HF encourage greater involvement in their programs of experienced sleep physicians and sleep laboratories with demonstrated capacity to discriminate between OSA and CSA using contemporary diagnostic standards (Weak Recommendation; Moderate-Quality Evidence).
129. We recommend continuous positive airway pressure (CPAP) for symptom relief for patients with HF with OSA either who are limited by daytime hypersomnolence (Strong Recommendation; Moderate-Quality Evidence) or whose OSA initiates arrhythmias including AF (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
SDB treatment is complex and ongoing trials, including the ADVENT-HF trial will improve the knowledge of how best to treat patients with HF. This recommendation takes into account the value of treating OSA and the cost of diagnosis and treatment for OSA and/or CSA.
Practical Tip
Because the prevalence of coexisting OSA and CSA in patients managed by HF programs remains > 50% despite contemporary medical and device therapy and because most patients with HF with sleep apnea do not complain of daytime sleepiness, their evaluation should include inquiry from sleep partners into witnessed apneas, airway obstruction, and oscillating breathing patterns during sleep.
Consider OSA in patients with HF who present with paroxysmal or recurrent AF, hypertension refractory to optimal HF therapy, high body mass index, and unanticipated pulmonary hypertension or RV dysfunction.
Consider the coexistence of sleep-related breathing disorders in patients with HF when malignant ventricular arrhythmias are detected, particularly at night.
7.4 Acute heart failure
7.4.1 Diagnosis, evaluations, and investigation
The diagnosis of AHF is on the basis of a constellation of symptoms (eg, orthopnea and shortness of breath on exertion) and signs (eg, edema and respiratory crackles) and supported by targeted investigations.[404],[405] Physical examination evaluates systemic perfusion and presence of congestion.[182],[405]–[407] Laboratory testing, ECG, chest x-ray, and ECHO are all important to obtain relatively efficiently.[406]
Clinicians should consider potential etiology and precipitating factors (Table 3). In many cases (75%-80%), a precipitant can be found (Table 25).[408],[409] Failure to uncover the responsible precipitating factor might lead to intractable HF. Noncompliance with diet or medication intake, infections, arrhythmias, pulmonary embolism, and ACS are frequent situations that might cause AHF.
It is essential to perform an ECG in AHF, although it might sometimes be normal. It assists in identifying rhythm abnormalities (AF, flutter, or bradycardia and ventricular tachycardia), ACS,[410] RV, LV, or atrial hypertrophy or strain, and myopericarditis. Cardiac arrhythmias should be evaluated using a 12-lead ECG and continuous ECG monitoring. A chest x-ray should also be performed in all patients with suspected AHF within the first 1-2 hours of arrival to assess cardiac size and shape, pulmonary congestion, and other pulmonary conditions (Fig. 8).
The utility of NPs to exclude (“rule out”) or confirm (“rule in”) the diagnosis in the appropriate clinical scenario is well established and discussed in section 6. Biomarkers/ NPs. 57,[406],[411],[412] Several clinical scoring systems have been derived and validated and combine commonly used clinical features with NP values to improve diagnosis and decisionmaking (Table 26).[413],[414] One such clinical scoring system) was developed from the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) study.[413]
Recommendation
130. We suggest the use of a validated diagnostic scoring system for patients in whom the diagnosis of AHF is being considered (Weak Recommendation; Low-Quality Evidence).
131. We recommend the diagnosis of AHF be established within < 2 hours of the initial contact in the ED (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation places a relatively high value on evaluating the constellation of clinical findings in a patient with suspected AHF and less value on an individual physical examination finding, presenting symptom, or investigation.
Practical Tip
A precipitating cause for AHF should be sought.
An ECG, blood tests, and a chest x-ray should be performed within 2 hours of initial presentation.
Initial blood tests should include: complete blood count, creatinine, blood urea nitrogen, glucose, sodium, potassium, and troponin.
A transthoracic ECHO should be performed within 72 hours of presentation. For patients with a previous ECHO, another is not required unless there has been a significant change in clinical status requiring investigation, a lack of clinical response to appropriate therapy, and/or it is > 12 months since the previous ECHO.
7.4.2 Initial and ongoing treatment
Oxygen should be used cautiously in normoxic patients because of concerns of increasing systemic vascular resistance and reducing cardiac output.[415],[416] There is a paucity of evidence to support the use of I.V. morphine to treat dyspnea, and data suggest there might be adverse effects, even after accounting for the severity of illness, comorbid conditions, and cointerventions (Fig. 9).[417]–[419]
Recommendation
132. We recommend supplemental oxygen be considered for patients who are hypoxemic; titrated to an oxygen saturation > 90% (Strong Recommendation; Moderate-Quality Evidence).
133. We recommend that morphine not be used routinely in patients with AHF (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation places higher value on the physiologic studies showing potential harm with the use of excess oxygen in normoxic patients and less value on long-term clinical usage of supplemental oxygen without supportive data.
Values and Preferences
This recommendation places higher value on large epidemiological studies with appropriate methods showing harm with the use of morphine in patients with AHF.
BiPAP or CPAP should be considered for patients with a high respiratory rate (eg, > 25 breaths per minute) and persistent systemic arterial hypoxemia despite high-flow oxygen administration.[420],[421] However, routine use of noninvasive ventilation (NIV) is not advisable. In the Three Interventions in Cardiogenic Pulmonary Oedema (3CPO) trial,[417] patients with acute pulmonary edema were randomized to standard oxygen therapy, CPAP, or NIV, and followed to the primary end point of 7-day mortality. There was no difference between the 3 arms on 7-day mortality rate and 30-day mortality rates, intubation rates, or admission to an intensive care unit. Therefore NIV should be used only in patients with acute respiratory distress unresponsive to medical therapy. NIV carries the risk of worsening RHF, hypercapnia, aspiration, and pneumothorax. Endotracheal intubation might be used if less invasive modes of oxygen delivery fail or if the patient is in cardiogenic shock.
Recommendation
134. We recommend that CPAP or bilevel positive airway pressure (BiPAP) not be used routinely in/for patients with AHF (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation places high weight on RCT data with a demonstrated lack of efficacy and with safety concerns in routine use. Treatment with BiPAP/CPAP might be appropriate for patients with persistent hypoxia (peripheral O2 saturation < 90%), high respiratory rate (> 25 breaths per minute), and pulmonary edema despite other appropriate therapies.
Oral and I.V. diuretics remain the mainstay of early therapy directed toward AHF (Table 27).[422] Diuretics generally lead to excretion of sodium and water, leading to a decrease in extracellular fluid volume, total body water, and sodium. A reduction in cardiac filling pressures, peripheral congestion, and pulmonary edema usually follow.[423] I.V. loop diuretics might cause an early decrease in right atrial pressure and PCWP. When using high I.V. doses reflex vasoconstriction might occur. In AHF, by normalizing loading conditions, these high doses might reduce neurohormonal activation in the short-term.[424]
Diuretic therapy may be initiated in the ambulance,[425] HF clinic, or in-hospital. Combining loop diuretics with thiazides[426],[427] or spironolactone[428] has been proposed and appears effective, with fewer side effects than a higher dose of a loop diuretic. In patients with severe edema, oral loop diuretics might not be adequately absorbed and might be of little use.[406] Using stepped pharmacologic diuretic therapy is a useful approach and has been used as the control arm in the CARRESS-HF trial (Fig. 10).[394]
The Diuretic Optimization Strategies Evaluation (DOSE) trial enrolled 308 patients with AHF and tested 2 I.V. strategies (high- vs low-dose furosemide; continuous infusion vs bolus intermittent dose) for the primary end point of global symptom assessment and creatinine at 72 hours.[422] There was no difference between the continuous infusion and bolus dosing in either symptoms or renal function. There was greater early symptom improvement with high- compared with low-dose diuretics without a difference in renal function. A number of secondary end points favoured high-dose: a greater diuresis, more weight loss, and a lower NT-proBNP resultant level. A systematic review of 9 additional small trials showed similar findings (Fig. 11).[429] Thus, there is no advantage in the routine use of continuous diuretic infusions and a higher dose of diuretics could be considered for many patients, with careful observation of renal function and electrolytes. Diuretic responsiveness is another consideration and metrics for the re-evaluation of a patient’s individual response have been reported.[425],[431] For example, weight loss (or urine output) per diuretic dose unit have been retrospectively evaluated to identify patients with a poor response to diuretics (over 1 day losing < 0.4 kg per 40 mg furosemide) as those at higher risk for short- and long-term morbidity.[430]
Recommendation
135. We recommend that I.V. diuretics be given as firstline therapy for patients with pulmonary or peripheral congestion (Strong Recommendation; Low-Quality Evidence).
136. We recommend that for patients requiring I.V. diuretic therapy, furosemide may be dosed intermittently (eg, twice daily) or as a continuous infusion (Strong Recommendation; Moderate-Quality Evidence).
Practical Tip
When acute congestion is cleared, the lowest dose that is compatible with stable signs and symptoms should be used.
Target 0.5-1.0 kg of weight loss per 24-hour period while a patient with volume overload is actively diuresing. Patients who are losing < 0.5 kg per day despite at least 40 mg of I.V. furosemide will need a reassessment of fluid status and might be diuretic resistant.
When transitioned from I.V. to oral diuretic therapy, the stability of symptoms, weight, and hemodynamics should be observed for approximately 24 hours before hospital discharge.
To transition a patient to oral diuretics, be aware that the oral version of furosemide has approximately 50% bioavailability compared with I.V. furosemide.
Vasodilators have not been shown to convincingly reduce mortality or reduce rehospitalization rates.[432] I.V. isosorbide dinitrate (in conjunction with low-dose furosemide) was tested against low-dose nitrates with high-dose diuretics.[433] This prehospital trial of 110 patients showed that the strategy of high-dose nitroglycerin (compared with high-dose I.V. diuretics) reduced mechanical ventilation rates, and improved oxygen saturation. The Vasodilatation in the Management of Acute CHF (VMAC) trial compared nesiritide, nitroglycerin, or placebo combined with standard therapy for 3 hours, followed by nesiritide or nitroglycerin combined with standard treatment for 24 hours in AHF.[434] The primary end points of changes in PCWP and patient self-evaluation of dyspnea at 3 hours were improved with nesiritide vs placebo. However, nitroglycerin improved early, short-term dyspnea assessment compared with placebo. The Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure (ASCEND-HF) trial tested nesiritide vs placebo in 7141 patients with AHF enrolled within 24 hours of first I.V. medication.[435] Nesiritide did not reduce mortality, rehospitalization, or the composite of these end points at 30 days. Dyspnea was modestly improved at 6 and 24 hours. The use of nitroprusside in AHF has not been supported by adequately powered RCTs. However, observational studies support its use in advanced HF by clinicians with experience and expertise in managing low-output acute or sub-AHF.[436] I.V. serelaxin, a vasodilator, was tested in the Preliminary Study of Relaxin for the Treatment of Acute Heart Failure? (PRE-RELAX) trial of 234 patients and had a modest improvement in dyspnea compared with placebo. The Relaxin for the Treatment of Acute Heart Failure (RELAX-AHF) trial tested serelaxin vs placebo in 1161 patients with AHF with a SBP > 125 mm Hg and enrolled within 16 hours of attending the ED. Serelaxin reduced dyspnea measured using a visual analogue scale over 5 days but not using a Likert scale over 24 hours. There was no reduction in the secondary end points of cardiovascular death, hospitalization for heart or renal failure, or days alive out of hospital up to 60 days, but a reduction in mortality at 180 days was seen. There are ongoing trials of serelaxin. There was no clinically meaningful improvement in outcomes with early upfront use of ularitide or TRV-027.[437],[438]
Recommendation
137. We recommend the following I.V. vasodilators for relief of dyspnea in hemodynamically stable patients (systolic blood pressure [SBP] > 100 mm Hg):
i. Nitroglycerin (Weak Recommendation; Moderate-Quality Evidence);
ii. Nesiritide (Weak Recommendation; HighQuality Evidence); or
iii. Nitroprusside (Weak Recommendation; Very Low-Quality Evidence).
Values and Preferences
This recommendation places a high value on the relief of the symptom of dyspnea and less value on the lack of efficacy of vasodilators or diuretics to reduce hospitalization or mortality.
Practical Tip
In situations in which I.V. nitroglycerin is not appropriate or available, repeated sublingual nitroglycerin, a nitroglycerin patch, or oral isosorbide dinitrate might be useful for dyspnea relief in patients with a SBP > 100 mm Hg.
Inotropic agents have not been shown to improve patient outcomes.[406],[439]–[441] The Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) trial randomized 951 patients admitted for HF to a 48-hour infusion of milrinone or placebo.[440] New-onset atrial arrhythmias, worsening HF, and symptomatic hypotension requiring intervention occurred more frequently in the milrinone group. A nonsignificant increase in the number of deaths in-hospital and after 60 days was seen in the milrinone group. A post hoc analysis showed a higher incidence of death or rehospitalization in patients with underlying ischemic HF etiology.[441] Trials using levosimendan have not shown additional benefit compared with placebo[442]; omecamtiv mecarbil is undergoing further testing in a RCT.[443] Low-dose dopamine has been studied in the context of AHF[444],[445] and does not improve clinical symptoms, renal function, or reduce clinical events.
Recommendation
138. We recommend that hemodynamically stable patients not routinely receive inotropes like dobutamine, dopamine, levosimendan, or milrinone (Strong Recommendation; High-Quality Evidence).
Values and Preferences
This recommendation for inotropes places a high value on the potential harm shown when systematically studied in clinical trials and less value on potential short-term hemodynamic effects of inotropes.
Practical Tip
I.V. vasoconstrictor agents (eg, phenylephrine, norepinephrine) should generally be avoided for AHF management except for hypotensive patients with SBP < 90 mm Hg, associated signs or symptoms, end-organ damage, and a significant change from baseline.
In patients with low SBP (< 90 mm Hg), low cardiac output and either euvolemia or hypervolemia, inotropes may be used for stabilization. An ACEi (or ARB) should not be started as de novo therapy in the acute setting (eg, the first 8-12 hours) unless an elevated SBP is present, but should be initiated after the acute event (eg, > 24 hours), and be continued particularly if the patient is already being treated with chronic ACEi or ARB therapy. There are no data on initiating an ARNI in this situation.
Continuation of a β-blocker upon admission for AHF is considered safe on the basis of the limited data available, including patients receiving inotropes.[446],[447] In an RCT of 169 patients with AHF, patients either discontinued β-blockade for 3 days or continued the medication unchanged. The trial showed that continuing the β-blocker was noninferior for the primary end point of dyspnea and wellbeing and was associated with a higher rate of β-blocker prescription at 3 months.[447]
Recommendation
139. We recommend continuation of chronic β-blocker therapy in a patient with AHF, unless the patient is symptomatic from hypotension or bradycardia (Strong Recommendation; Moderate-Quality Evidence).
Practical Tip
A major reduction in dose or abrupt β-blocker withdrawal should be avoided in the case of worsening HF. If the patient is hypotensive, consider reducing the dose of other medications before reducing the β-blocker dosage. Temporary discontinuation might occasionally be necessary in patients with shock. Whenever possible, reinstitution of treatment should be attempted before hospital discharge.
Vasopressin receptor antagonists (eg, tolvaptan) can rapidly and effectively reduce body weight and restore serum sodium in patients with significant symptomatic hyponatremia with hypervolemia and congestion.[448],[449] The use of a vasopressin antagonist has not yet been associated with mortality or rehospitalization reduction.[450] A subgroup of 11% and 3% of the patients in the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial had a serum sodium < 135 mmol/L and < 130 mmol/L respectively, and in post hoc analysis, the latter patients had an association with fewer clinical events when treated with tolvaptan.[451]
Recommendation
140. We suggest that tolvaptan be considered for patients with volume overload, hyponatremia (< 130 mmol/L), and symptoms of hyponatremia for the short-term correction of hyponatremia and associated symptoms (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation places higher value on the correction of symptoms and complications related to hyponatremia and less value on the efficacy of vasopressin antagonists to reduce HF-related hospitalizations or mortality.
7.4.3 Initial and ongoing monitoring and disposition decisions
The extent of monitoring will depend on the disease severity and the response to therapy.[406] Vital signs (including BP, heart rate, O2 saturation, and daily weight) should be measured on a regular basis until stabilization. Laboratory tests should be repeated regularly (eg, daily in the first 2-3 days): electrolytes, creatinine, and complete blood count, if abnormal. Electrolyte abnormalities, especially hypokalemia, which has been linked to ventricular arrhythmias,[452] should be prevented or corrected promptly. There is limited evidence to support measurement or replacement of magnesium in patients with AHF. Significant renal impairment might require more frequent laboratory testing.
Decisions to admit a patient from the ED to hospital are complex and require integration of the patient’s clinical stability and preferences and health system features including the availability of appropriate outpatient follow-up. In Canada, 60%-80% of patients in the ED are admitted with AHF.[446] Whereas there are HF risk models to determine overall mortality risk, there are few instruments to guide clinicians which patients need admission to hospital, continued observation, or discharge home with close follow-up.[453] One instrument developed from prospectively collected data on 1033 patients with AHF created a nomogram for predicting 5- and 30-day clinical events.[454] Using different risk of future event thresholds, the nomogram identified a group of patients potentially eligible for safe ED discharge. Two other Canadian studies have developed prognostic risk scores that are as yet tested in RCTs. Table 28 highlights key considerations for these decisions; also see Table 29.
Clinical deterioration despite initial therapy requires closer supervision, such as transfer to an intensive care unit.[455] Patients in cardiogenic shock or those who have difficulty voiding should have a urinary catheter to monitor urinary output; however, recording of “ins/outs” (also known as fluid balance) is not necessary in all clinically stable patients and can require significant resources to obtain accurately.[406] The decision to insert an arterial line depends on the need for either continuous analysis of BP because of hemodynamic instability or the requirement for repeated arterial blood gas analyses.[406] The use of a central I.V. line depends on the need for delivery of fluids and drugs or for monitoring central venous pressure and oxygen saturation. However, in the critically ill, right atrial pressure does not correlate well with left-sided filling pressures.[456] The insertion of a pulmonary artery catheter is not usually necessary for making a diagnosis or ongoing management of AHF.[457]–[459] It might, however, be useful to distinguish between cardiogenic and noncardiogenic shock, to guide therapy in the presence of severe diffuse pulmonary disease, or in hemodynamically unstable patients who do not respond in a predictable fashion to therapy.[460]
Recommendation
141. We recommend that a pulmonary artery catheter not be used routinely in patients with AHF (Strong Recommendation; Moderate-Quality Evidence).
Practical Tip
Tailored hemodynamic therapy with a pulmonary artery catheter under experienced supervision might be clinically useful in highly selected cases, such as ongoing HF accompanied by CRS, poor response to therapy or systemic hypotension, or as evaluation for advanced therapies (MCS or heart transplantation).
7.5 Special circumstances
7.5.1 Cardiomyopathies
7.5.1.1 HCM
HCM is a disease of the myocardium characterized by pathological and disproportionate hypertrophy of the left ventricle and, sometimes, the right ventricle. It is most often an inherited condition with an autosomal dominant pattern with variable penetrance. The onset of HCM can occur in early as well as late adulthood,[461] with a risk of sudden death, often at a young age. Most patients with HCM are asymptomatic.[462] Symptoms and signs might include chest pain, dyspnea, palpitations, or syncope. Symptoms can arise as a consequence of LV outflow tract obstruction (with secondary MR), tachyarrhythmia (especially ventricular tachycardia and AF), and myocardial ischemia; chest pain is common, even in the absence of CAD[463] and impaired diastolic function or systolic dysfunction. In a small percentage of cases, the disease progresses to a burnt-out phase, with the development of severe systolic dysfunction that resembles dilated cardiomyopathy (Table 30).[463],[464]
HCM should be suspected in any individual who presents with unexplained ventricular hypertrophy, heart murmurs (from dynamic outflow obstruction), abnormal ECG patterns (pseudoinfarction, giant negative T waves), unexplained syncope (particularly in young athletes), or a positive family history. A number of hereditary syndromes (including Friedreich ataxia,[465] and LEOPARD syndrome[466]) have also been linked to HCM. Approximately 30% of the cases are diagnosed de novo in elderly patients.[467]
Transthoracic echocardiography (with or without contrast) is the imaging modality of choice, and might include a provocative test for dynamic LV outflow tract obstruction. CMR imaging may be used if initial imaging results are nondiagnostic and might provide other structural details.[468] Tissue Doppler imaging might aid in early diagnosis.[469] Localized hypertrophy of the interventricular septum is the most common pattern of hypertrophy, but other patterns are also possible. Dynamic LV outflow obstruction occurs in approximately 30%-50% of cases and might be latent.[470] Differentiation must be made from other pathologies that mimic HCM in appearance, including concentric hypertrophy due to systemic hypertension, physiological hypertrophy seen in trained athletes, and discrete/disproportionate upper septal hypertrophy in elderly patients.
Practical Tip
All first-degree relatives of patients with HCM should be screened for the disease with an ECG and echocardiography.
HF symptoms in patients with HCM might be due to diastolic dysfunction (most common), outflow tract obstruction (less common), rhythm disturbance, or concomitant valvular or ischemic heart disease (Table 30).
7.5.1.2 Restrictive cardiomyopathy and constriction
Restrictive cardiomyopathy (RCM) as the etiology of HF can be difficult to recognize and is the least common category among the cardiomyopathies.[471],[472] It is characterized by myocardium with markedly stiff ventricular walls, restrictive ventricular filling, and reduced diastolic volume of either or both ventricles, and normal or near-normal systolic function.[472],[473] Myocardial fibrosis, infiltration, or endomyocardial scarring is responsible for the diastolic dysfunction.[473] Consequently, RCM shares similar functional characteristics with constrictive pericarditis, and differentiation between the two might be difficult but imperative because surgery might potentially cure the latter.[471] Amyloidosis is a frequent cause, but many other situations might also result in RCM. Rare hereditary forms of RCM have been described, such as troponin I gene mutations or in association with skeletal muscle disease (Table 31).[473],[474] Prognosis of patients varies substantially, but it is generally one of inevitable downward symptomatic progression with a high mortality.[475]
7.5.1.2.1 Specific imaging and diagnostic tests for restrictive cardiomyopathies and constriction
There are a number of selected tests that will aid either the diagnosis, prognosis, or a selection of appropriate therapy for patients with a suspected RCM or constriction. These are presented and discussed in the following sections. Not all laboratory or imaging tests are readily available in all regions, and therefore, expertise should be sought where necessary.
7.5.1.2.2 Physical examination and ECG
Patients usually exhibit typical symptoms and signs of HF, including Kussmaul sign that might be disproportionate to the degree of systolic or valvular dysfunction. Hepatomegaly, ascites and, in more advanced cases, anasarca, might be present. Notably, the apex beat is usually palpable in RCM, but not in constrictive pericarditis.[473] The ECG is frequently abnormal with decreased voltage intraventricular conduction delay, or poor R wave progression mimicking MI.[476] Sinus node disease is frequent, and typical characteristics of the sick sinus syndrome are encountered.[477] Arrhythmias, mostly AF, are frequent and might be caused by amyloid deposition.[478]
7.5.1.2.3 Laboratory findings
Specific morphological features (especially musculoskeletal) in the setting of renal disease and a family history of metabolic abnormalities suggest the presence of an infiltrative disorder, such as Fabry disease. Fabry disease is a rare X-linked disorder that can present with neuropathy, renal failure, and HF in affected men and hemizygous women. Genetic testing can determine the exact gene defect, but elevated urinary globotriaosylceramide, and reduced plasma a-galactosidase activity are diagnostic.[479] These disorders usually require tissue diagnosis, either from cardiac tissue or other affected organs, such as the kidneys.
Abnormalities in serum protein electrophoresis suggest amyloidosis, whereas a high plasma level of ferritin, in combination with increased transferrin saturation, suggests hemochromatosis. Ventricular arrhythmias might be a forerunner for sudden death, and a signal-averaged ECG has been suggested to help in identifying patients at risk.[480]
An abdominal fat aspirate is safe and might assist in the diagnosis of amyloidosis. If the result of an abdominal fat aspirate is negative, EMB might help if cardiac amyloidosis is suspected, and kidney biopsy might also be useful if the eGFR is low, suggesting renal involvement. Immunohistochemical staining should be performed because it helps to differentiate between systemic senile, familial, and primary forms of amyloidosis, and clarifies prognosis and management, which differ in the various forms.[481],[482] Unsuspected hereditary amyloidosis might be present in nearly 10% of patients thought to have the primary amyloid light-chain form.[483] The absence of concomitant symptoms and signs of systemic illness, nonspecific laboratory findings, and negative EMB strongly suggest idiopathic RCM.
7.5.1.2.4 Imaging
Echocardiography might be normal early on, but small ventricular chambers with increased wall thickness, large atria, and thickening of valvular apparatus and interatrial septum are often seen. LV systolic function is usually normal, but might be reduced in advanced disease. Pericardial effusion is common, but rarely results in tamponade.[484] The sparkling appearance of the thickened walls, presumably related to amyloid deposition, was previously believed to be typical on echocardiography, but is less reliable compared with recent technology.[485] Doppler echocardiography is useful for the diagnosis, prognosis, and should be evaluated carefully in centres with experience and expertise.[486],[487]
CMR can precisely image functional, morphological changes,[488] fibrosis,[489] and inflammation[490] and might provide early insight into the disease process and etiology. CMR is useful in amyloidosis,[491] with subendocardial contrast enhancement associated with nonsuppressible signal of remote myocardium. CMR might increase the sensitivity of myocardial biopsy,[492] and might help to direct treatment.[493] CMR might be helpful in distinguishing between active myocardial disease and clinical remission in systemic lupus erythematosus,[494] documenting the presence of cardiac fibrosis in Churg-Strauss syndrome, a rare form of systemic vasculitis,[495] and detecting early indications of iron overload in thalassemia patients.[496]
Scintigraphy with technetium-99m pyrophosphate, as well as with other agents that bind to calcium, is frequently positive, with extensive amyloid infiltration. Scanning with specialized agents might also identify sympathetic denervation in patients with cardiac amyloidosis.[497]
7.5.1.2.5 Hemodynamic findings
The characteristic hemodynamic feature of RCM as well as constrictive pericarditis on cardiac catheterization is a rapid early decrease in ventricular pressure at the beginning of diastole, with a rapid increase to a plateau in early diastole (this latter finding might be absent in RCM)dthe ‘dip and plateau’ or ‘square root’ sign. Systemic as well as pulmonary venous pressures are elevated (frequently > 50 mm Hg in RCM, although lower in constrictive pericarditis), and patients with RCM typically have LV filling pressure that exceeds RV filling pressure by more than 5 mm Hg (this difference can be revealed by exercise, fluid challenge, or the Valsalva manoeuvre). In constrictive pericarditis, filling pressures are similar in both ventricles, and the plateau of the RV diastolic pressure is usually at least one-third of the peak RV systolic pressure, but is frequently lower in RCM. In the setting of rapid early diastolic ventricular filling, an increase in RV peak systolic pressure during inspiration might occur with a reduction of LV peak systolic pressure.
Differentiating between RCM and constrictive pericarditis might be difficult and thus multiple modalities in centres experienced with these techniques is important. EMB and CT might also be useful for differential diagnosis; however, in rare cases, open biopsy might be required.[471]
Practical Tip
Radionuclide ventriculography, CMR and/ or echocardiography can be used to noninvasively determine RV EF and other measures of RV function.
7.5.2 Ethnicity
On the basis of a Statistics Canada 2006 census,[498] the visible minority population surpassed 5 million, reaching 16.2% of the population. In Ontario, more than 1.5 million people are of Chinese, South Asian, black, or Aboriginal descent. To understand and manage a person’s illness it is necessary to appreciate the effects of the person’s culture and social environment. This is perhaps most relevant in the health care management of minority groups. Morale is crucial to the patients’ adaptation and their maintenance of involvement in their management; miscommunications as a result of ethnocultural differences might have a detrimental effect on their adaptation to their illness. Furthermore, health care providers might contribute to the ethnic care disparities through clinical uncertainty and stereotyping of health behaviours related to minority patients.[499] However, the use ethnicity as a way to differentiate patients might be debatable and differential medical treatment on the basis of the colour of one’s skin has been associated with detrimental outcomes for ethnic minorities.[500] A significant contributor is the paucity of research to clearly identify the sources of these differences in outcomes in ethnic groups and to distinguish among biological, environmental, or social causes of disease differences.[501] Evaluation of disease differences in subsegments of the population is needed to understand the mechanisms of pathophysiology and to optimally target therapeutic responses. Thus, effective research that would contribute to a reduction in health care disparities requires collection of data on health status in ethnic populations and assessment of differences in disease patterns. It also requires clinical trials to include adequate numbers of diverse populations to probe for differences in pathophysiology including environmental or social factors contributing to disease and responses to treatment. Where differences are observed among population segments, clinical trials focused in these population groups are warranted.[501],[502]
7.5.2.1 General considerations
There have been very few published population-based epidemiological studies or large-scale randomized controlled studies of HF in countries outside North America, Europe, and Australia,[503] regions from which most of the minority groups that reside in the Western countries emigrated. For example, it is generally believed that rheumatic heart disease and congenital heart disease remain important causes of HF in sub-Saharan Africa and certain parts of Asia and South America. Hypertension is thought to be an important cause of HF in Asia, and in the African and African American population, whereas Chagas disease is an important etiology in subjects from South America.[504] Although it is useful to remember region-specific etiologies of HF particularly when managing recent immigrants from the regions where the minority groups were resident, it should be remembered that because these regions also constantly undergo epidemiological and economic transitions the epidemiology of HF is likely to be increasing similar to that of the Western world. The INTERHEART study has shown that the effect of conventional and potentially preventable risk factors on the risk of MIs are consistent across different geographical regions and different ethnic groups.[505] This implies that simple measures that can prevent MI and likely the subsequent development of HF are equally applicable to different ethnic populations in different geographic locations. The recently published Cardiovascular Health in Ambulatory Care Research Team (CANHEART) Immigrant study[506] shows that most immigrant groups in Canada have lower rates of major cardiovascular events than long-term residents of similar age; striking variations in the event rates exist between immigrants from different ethnic background. East Asian immigrants, predominantly of Chinese descent, had the lowest burden of risk factors and events overall, although the event rate increased with greater duration in Canada. There are also high-risk groups such as South Asian immigrants, who had a high burden of traditional risk factors and frequent cardiovascular events. In general, patients in the Asia Pacific region have historically less CAD as etiology, onset at younger age, fewer uses of devices, more diabetes, and more uses of parenteral agents during acute episodes.[507]
There is little evidence to indicate that criteria used to diagnose HF differ between ethnic populations. For example, a recent study from the United States has shown that the diagnostic performance of the biomarker N-terminal BNP is similar in African American and non-African American individuals.[508] Evidence that different ethnic groups have the same mortality benefit from current standard therapy is scant because very few large RCTs have included regions outside Europe and the United States. There have been smaller trials that confirmed the effectiveness of ACEis and β-blockers in patients from Africa and Asia.[504],[509] Because of the fundamental nature of the derangements in HF, it is likely that the current treatment approach such as blockade of neurohormonal activation and the judicious use of devices will be similarly effective, although one cannot rule out the possibility that the degree of response to treatment might vary among ethnic groups. Details that pertain to specific minority populations are available in section 7.5.2.2 of the Supplementary Material. Recommendations and practical tips on the management of patients with HF from the 4 largest ethnic minority groups in Canada are shown in Table 32.
7.5.3 Pregnancy
HF during pregnancy has a number of potential etiologies including PPCM. Preconception counselling is recommended in women with inheritable and/or a known history of HF or PPCM. Maternal risk assessment and frequency of expert follow-up should be scheduled as recommended by the modified WHO risk classification.[510],[511] High-risk patients should be referred to a multidisciplinary team with expertise in the management of HF and highrisk pregnancy.[512]–[514]
7.5.3.1 Diagnosis and management
Hemodynamic changes in normal pregnancy can precipitate HF in patients who are susceptible or have another underlying etiology (Table 33). Decompensation can occur at any time; however it is more common in the late stages of pregnancy and peripartum. Physical examination for HFrelated physical signs can be challenging in pregnancy. It is important for clinicians to recognize cardiovascular symptoms and signs that are not normally present in pregnancy (Table 34). Echocardiography remains the preferred imaging modality for HF during pregnancy. Women with AHF during pregnancy should be managed according to the guidelines for AHF and should be referred to a tertiary care centre with expertise in advanced HF management, including MCS and cardiac transplantation.[515],[516]
7.5.3.2 Medical therapy of HF in pregnancy
HF in pregnancy should be treated according to the CCS HF guidelines for acute and chronic HF. However, many standard therapies of HF are considered fetotoxic and should not be used during pregnancy including ACEis, ARBs, and MRAs.[517],[518] There are no data on the use of ARNIs and thus they are not recommended during pregnancy. When β blockade is used, β1-selective drugs (eg, metoprolol) should be preferred. Atenolol should not be used.[519] Diuretics can be used if pulmonary congestion is present. Medications that might be used for pregnant women with HF are shown in Table 35. An additional comprehensive list of medications is available at www. motherrisk.org.[515],[516]
7.5.3.3 PPCM
PPCM is a diagnosis of exclusion typically defined as HF with an LVEF of < 45% within 1 month before delivery to 5 months postpartum. Pathophysiologic mechanisms are not clearly defined but might include genetic predisposition,[520] oxidative stress, and immune mechanisms,[521],[522] as well as viral infections[521] and prolactin.[523] Risk factors for the developments of PPCM include multiparity and multiple fetal gestation, advanced maternal age, family history, ethnicity, hypertension, preeclampsia, smoking, diabetes, and prolonged tocolytic therapy.[524]
In addition to standard diagnostic tests for HF and pregnancy, there is now data to support the use of biomarkers for diagnosis as well as prognosis in PPCM.[525],[526] There have been several case reports and a small RCT to evaluate bromocriptine[527] with inconclusive results and uncertain safety.[528]–[530]
Despite advances in HF treatment mortality as well as morbidity related to PPCM remains high. In case series, LV systolic function returns to normal in 23%-72% of patients.[524],[531]–[533] Less is known about the risk of subsequent pregnancy; however, the 2 largest studies of PPCM suggest relapse occurs in almost one-third of the cases.[534],[535] Thus most would agree that individualized counselling should occur in individuals with PPCM and they should be advised against future pregnancy particularly if recovery of LVEF has not occurred.[524],[536]
Recommendation
142. We recommend that pregnant women (or those in the peripartum period) with AHF should be managed according to the CCS guidelines for AHF and should be referred to a tertiary centre with expertise in advanced HF management, including MCS and cardiac transplantation (Strong Recommendation; Low-Quality Evidence).
143. We recommend that NPs be used for diagnostic and prognostic purposes in peripartum cardiomyopathy (PPCM) (Strong Recommendation; Low-Quality Evidence).
144. We recommend that bromocriptine not be used routinely for PPCM (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
Adequately powered and appropriately designed RCTs have not been completed. The safety of bromocriptine is not well established.
145. We recommend that echocardiography be performed in women with worsening or suspected new-onset HF during pregnancy (Strong Recommendation; Low-Quality Evidence).
146. We recommend prepregnancy counselling in all women with a known history of HF or PPCM (Strong Recommendation; Low-Quality Evidence).
147. We recommend preconception genetic counselling in women with inheritable cardiac diseases that can affect cardiac function, including inheritable cardiomyopathies (Strong Recommendation; Low-Quality Evidence).
148. We recommend maternal risk assessment and frequency of expert follow-up should be determined using the modified WHO risk classification (Strong Recommendation; Low-Quality Evidence).
149. We recommend that decisions regarding timing and mode of delivery should be on the basis of obstetrical factors (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
Cesarean deliveries are not routinely necessary and might additional risk to patients with HF. Delivery before term for cardiac decompensation is rarely required.
Practical Tip
Vaginal delivery is preferred in women with stable cardiac conditions.
Recommendation
150. We recommend that patients with PPCM who do not recover normal LV function should be advised against future pregnancies because of the high risk of worsening HF and death (Strong Recommendation; Moderate-Quality Evidence).
151. We recommend that patients with PPCM who recover normal LV function should be advised regarding the potential for recurrent LV dysfunction in subsequent pregnancies (Strong Recommendation; Moderate-Quality Evidence).
Practical Tip
The risk of thromboembolism associated with PPCM is increased because of the hypercoagulable state of pregnancy, and is highest during the first 6 weeks postpartum.
Recommendation
152. We recommend that several commonly used cardiac medications should be avoided because of teratogenic effects during pregnancy and with caution during lactation (Strong Recommendation; Moderate-Quality Evidence).
Practical Tip
Women with HF during pregnancy should be closely followed and monitored at the time of delivery and the early postpartum period.
Echocardiography is the preferred imaging modality for HF during pregnancy. Postpartum imaging can include CMR imaging for more accurate detection of changes in cardiac function and higher sensitivity for the detection of thrombus.
7.5.4 Cardio-oncology and HF
Cancer therapy might result in subclinical and clinical LV dysfunction. The CCS recently published guidelines for evaluation and management of cardiovascular complications of cancer therapy.[55] Specific recommendations, from that document, related to the development and treatment of LV dysfunction are presented in this document for ease of review; the content has not been modified. Readers are directed to the CCS guidelines for evaluation and management of cardiovascular complications of cancer therapy for a discussion of the evidence underpinning these recommendations. Our current understanding of agents associated with LV dysfunction, their mechanism, and time course for toxicity as well as patient- and treatment-related risk factors are summarized in Table 36. A comprehensive table of cancer treatments associated with all forms of cardiotoxicity are published elsewhere.[55] Additional information is also presented in section 7.5.4 of the Supplementary Material.
Recommendation
153. (CCS 2016 Cardio-oncology Recommendation 2): We recommend that patients who receive potentially cardiotoxic cancer therapy undergo evaluation of LVEF before initiation of cancer treatments known to cause impairment in LV function (Weak Recommendation; Moderate-Quality Evidence).
154. (CCS 2016 Cardio-oncology Recommendation 5): We suggest that serial use of cardiac biomarkers (eg, BNP, troponin) be considered for early detection of cardiotoxicity in cancer patients who receive cardiotoxic therapies implicated in the development of LV dysfunction (Weak Recommendation; Moderate-Quality Evidence).
155. (CCS 2016 Cardio-oncology Recommendation 6): We suggest that in patients deemed to be at high risk for cancer treatment-related LV dysfunction, an ACEi or ARB, and/or β-blocker, and/or statin be considered to reduce the risk of cardiotoxicity (Weak Recommendation; Moderate-Quality Evidence).
156. (CCS 2016 Cardio-oncology Recommendation 10): We recommend that in cancer patients who develop clinical HF or an asymptomatic decline in LVEF (eg, > 10% decrease in LVEF from baseline or LVEF < 53%) during or after treatment, investigations, and management, follow current CCS guidelines. Other causes of LV dysfunction should be excluded (Strong Recommendation; High-Quality Evidence).
157. (CCS 2016 Cardio-oncology Recommendation 12): We suggest that patients at high risk of cancer therapy-related CVD or patients who develop cardiovascular complications during cancer therapy (eg, > 10% decrease in LVEF from baseline or LVEF < 53%) be referred to a cardio-oncology clinic or practitioner skilled in the management of this patient population, for optimization of cardiac function and consideration of primary or secondary prevention strategies (Weak Recommendation; Low-Quality Evidence).
7.5.5 Myocarditis
Myocarditis, which often presents as HF, is an inflammatory process affecting the myocardium as a result of external antigen triggers such as viruses, bacteria, parasites, drugs, and others, or internal triggers such as autoimmune reaction to self-antigens. The WHO definition of myocarditis is on the basis of established histological, immunological, and immunohistochemical criteria.[537],[538]
The incidence of myocarditis is not well established because many patients with clinically suspected myocarditis do not undergo EMB or CMR. In patients with unexplained nonischemic cardiomyopathy, biopsy proven myocarditis was shown in 9%-16% of cases.[539],[540] The prognosis for patients with myocarditis is usually favourable. One prospective study suggested that approximately one-third of patients who present with acute myocarditis do not develop HF, one-third develop ventricular dysfunction with subsequent recovery, and approximately one-third are left with significant ventricular dysfunctionda small subgroup of subjects progressively deteriorate and require significant support (MCS or cardiac transplantation).[541],[542]
Clinical presentations of patients with myocarditis ranges from asymptomatic patients with abnormal ECG or ECHO findings to patients with atypical symptoms of fatigue, palpitations, chest pain at rest, and patients with arrhythmias, HF, cardiogenic shock, or sudden death. A high index of clinical suspicion along with biomarkers and noninvasive investigations like ECHO and CMR are necessary to make a diagnosis.
Markers of myocardial necrosis (troponin I or T) can assist in the diagnosis of myocarditis but a low or normal troponin level does not rule out myocarditis. The sensitivity of an elevated troponin I level in biopsy proven myocarditis in the Multicentre Myocarditis Treatment trial was 34% and specificity was 82%.[543] The ECG findings might include arrhythmias (ventricular or supraventricular), AV block, pattern of acute injury or pericarditis, nonspecific repolarization abnormalities or, rarely, might be normal. Echocardiographic findings might include segmental or global LV dysfunction, RV dysfunction, or pericardial effusion.[544]
CMR is the most important noninvasive investigation in the diagnostic workup of myocarditis. CMR allows for accurate and quantitative assessment of LV morphology, volumes, as well as global and regional ventricular function. More importantly however, CMR can be used to detect inflammation, by allowing visualization of myocardial hyperemia (early gadolinium enhancement), intracellular and interstitial edema (water-sensitive CMR), and necrosis/fibrosis (LGE) imaging. The combined protocol (Lake Louise criteria) has high specificity of up to 91% and a sensitivity of 67%. LGE imaging alone, however, also has a variable sensitivity of 44%- 100%[545] and is also not specific for acute vs chronic/healed myocarditis.[546]
In a study of 104 patients with subacute myocarditis, the extracellular volume with LGE imaging showed improvement in the diagnostic accuracy of CMR compared with standard Lake Louise criteria.[545] Myocardial mapping as a novel CMR approach might further improve diagnostic accuracy[547],[548]; further clinical research is ongoing.
The yield of EMB to provide clinically relevant information using Dallas criteria (histological criteria) is relatively low. Using immunohistochemical criteria and viral polymerase chain reaction in addition to histological criteria increases the diagnostic value of EMB.[549],[550] EMB should be considered if the results of biopsy are likely to result in a change of patient management. Giant-cell myocarditis is an example in which biopsy results provide information on prognosis and the need for immunosuppressive treatment. Other situations in which EMB provides unique information are disorders associated with sarcoidosis, hypereosinophilia, infiltrative cardiomyopathies, and others.
EMB is indicated for patients who present with an unexplained new onset of HF (< 2 weeks) and hemodynamic compromise. In this scenario, EMB might help establish diagnosis but has known safety considerations and diagnostic yield issues. EMB is also indicated in patients with new-onset HF associated with ventricular arrhythmias, high-degree AV blocks, and in patients who failed to respond to medical therapy. Ventricular arrhythmias and high-degree AV blocks are frequently associated with giant-cell myocarditis or sarcoidosis.[551]
Irrespective of clinical presentation, patients with myocarditis and HF should be treated with standard medical therapy for HF. The routine use of immunosuppression in myocarditis is not recommended. There are several small RCTs investigating the use of steroids alone or in combination with cyclosporine or azathioprine vs placebo in patients with myocarditis. In the largest study, 111 patients with biopsy proven myocarditis and reduced LV function (EF < 35%) were randomized to conventional therapy vs prednisone and either azathioprine or cyclosporine. At 12 months there was no survival benefit and no significant difference in LV function improvement.[539] In a study of 85 patients with biopsy proven virus-negative myocarditis, patients were assigned to placebo or prednisone in combination with azathioprine. At 6 months 88% of patients in the prednisone/azathioprine group showed significant improvement in echocardiographic parameters.[552] There are no large, randomized, placebocontrolled studies investigating antiviral therapy in patients with myocarditis. A high dose of I.V. immunoglobulin was evaluated in a small number of patients with myocarditis and the treatment was ineffective.[553]–[555]
Patients with known or suspected myocarditis should be referred to a centre where expertise in the diagnostic assessment and treatment of myocarditis is available. The urgency of referral is dependent on the clinical course. Reports of small case series suggest that aggressive medical support, including a ventricular assist device, along with medical therapy, has allowed for eventual ventricular function recovery and device explantation without the need for transplantation.[556],[557]
The intensity of follow-up for patients with myocarditis is dictated by the extent of cardiac dysfunction, the severity of the clinical presentation and the response to therapy. Followup consists of ongoing clinical assessment and might include echocardiographic assessment of cardiac function or CMR evaluation of ongoing inflammation. Emerging evidence suggests that CMR follow-up might be useful for predicting outcomes.[558] Persistent inflammation at 4-week follow-up indicates a worse prognosis in patients with no further inflammation. Patients with a positive clinical response to therapy, including improvement in or normalization of cardiac dysfunction, should also undergo clinical follow-up within approximately 3-6 months to confirm clinical stability. Patients with a continued or worsening course, which will be dictated by the clinical severity of symptoms and LV dysfunction, require ongoing expert follow-up.
Recommendation
158. We recommend that myocarditis should be suspected in the following clinical scenarios:
i. Cardiogenic shock due to LV systolic dysfunction (global or regional), in patients in whom etiology is not apparent.
ii. Acute or subacute development of LV systolic dysfunction (global or regional), in patients in whom etiology is not apparent.
iii. Evidence of myocardial damage not attributable to epicardial CAD or another cause (Strong Recommendation; Low-Quality Evidence).
159. We recommend referral to a centre with experience and expertise in the assessment and management of myocarditis should be considered for patients with suspected myocarditis (Strong Recommendation; Low-Quality Evidence).
160. We recommend that urgent referral for evaluation/ consideration for cardiac transplantation or MCS be considered for patients with myocarditis associated with HF, progressive clinical deterioration, or endorgan dysfunction despite standard HF therapy (Strong Recommendation; Low-Quality Evidence).
161. We recommend that all patients with suspected myocarditis have CMR where available and in the absence of contraindications (Strong Recommendation; High-Quality Evidence).
162. We suggest EMB be considered for patients who present with: (1) new-onset (< 2-week duration) HF of undetermined etiology with hemodynamic compromise; (2) HF and high-grade heart block; (3) HF with recurrent ventricular arrhythmias; or (4) HF unresponsive to medical therapy (Weak Recommendation; Low-Quality Evidence).
163. We recommend best medical therapy, including supportive care for the treatment of myocarditis (Strong Recommendation; Low-Quality Evidence).
164. We recommend against routine use of general or specific immunological therapies directed toward myocarditis, because this has not been shown to alter outcomes, and might lead to side effects or complications (Strong Recommendation; Moderate-Quality Evidence).
165. We suggest that treatment with immunosuppressive therapy should be considered in subgroups of patients with myocarditis due to specific underlying etiologies such as giant-cell myocarditis, sarcoidosis, myocarditis due to systemic autoimmune disease, or biopsy proven myocarditis with undetectable viral infection using polymerase chain reaction (Weak Recommendation; Low-Quality Evidence).
166. We recommend that antiviral therapy should not routinely be used in patients with myocarditis (Strong Recommendation; Low-Quality Evidence).
167. We recommend that expert clinical follow-up is required until myocarditis is determined to be resolved or until a chronic management plan is in place (Strong Recommendation; Low-Quality Evidence).
Practical Tip
Clinical signs and symptoms of myocarditis might be highly variable.
Other potential causes of cardiac dysfunction must be ruled out before a diagnosis of myocarditis can be made; additional tests might include cardiac catheterization or CMR, with or without RV biopsy.
Biomarker and 12-lead ECG findings in patients with myocarditis might mimic those of acute MI or acute pericarditis.
Patients with suspected myocarditis should have troponin I or T and BNP or NT-proBNP measured.
Treatment with immunosuppressive agents should be implemented by centres/physicians with considerable experience in managing these cases.
Patients with persistence of HF symptoms or ventricular dysfunction should be followed in a multidisciplinary HF/function clinic, and referred to specialized centres when appropriate.
Precise diagnostic criteria for acute myocarditis have not been prospectively validated; however, the criteria consider 4 major elements in determining the potential for the presence of acute myocarditis. They are:
- Symptoms and clinical findings consistent with acute or recent myocardial damage.
- Evidence of myocardial injury in the absence of a demonstrable epicardial coronary cause.
- Evidence of hyperemia, edema, or irreversible injury on CMR images.
- Presence of inflammatory cell infiltrate or positive viral genome signal on examination of EMB specimens.
Evaluation of EMB samples should be performed by an experienced cardiac pathology laboratory. Evaluation of EMB for myocarditis should include the use of histopathological markers of inflammation and necrosis, immunohistochemical markers, and assessment for viral particles.
References
93. Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992;327: 669-77.
94. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987;316:1429-35.
95. Kober L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med 1995;333: 1670-6.
96. SOLVD Investigators, Yusuf S, Pitt B, et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991;325:293-302.
97. Garg R, Yusuf S. Overview of randomized trials of angiotensinconverting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 1995;273:1450-6.
98. SOLVD Investigators, Yusuf S, Pitt B, et al. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions [erratum in 1992;327: 1768]. N Engl J Med 1992;327:685-91.
99. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet 1993;342:821-8.
100. Shibata MC, Flather MD, Wang D. Systematic review of the impact of beta blockers on mortality and hospital admissions in heart failure. Eur J Heart Fail 2001;3:351-7.
101. Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: the CAPRICORN randomised trial. Lancet 2001;357:1385-90.
102. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERITHF). Lancet 1999;353:2001-7.
103. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13.
104. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-8.
105. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893-906.
106. Cohn JN, Tognoni G, Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med 2001;345:1667-75.
107. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-Alternative trial. Lancet 2003;362:772-6.
108. Lee VC, Rhew DC, Dylan M, et al. Meta-analysis: angiotensin-receptor blockers in chronic heart failure and high-risk acute myocardial infarction. Ann Intern Med 2004;141:693-704.
109. Ezekowitz JA, McAlister FA. Aldosterone blockade and left ventricular dysfunction: a systematic review of randomized clinical trials. Eur Heart J 2009;30:469-77.
110. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364: 11-21.
111. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709-17.
112. Flather MD, Yusuf S, Kober L, et al. Long-term ACE-inhibitor therapy in patients with heart failure or left-ventricular dysfunction: a systematic overview of data from individual patients. ACE-Inhibitor Myocardial Infarction Collaborative Group. Lancet 2000;355:1575-81.
113. Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensinconverting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003;41:1529-38.
114. McMurray JJ, Ostergren J, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet 2003;362:767-71.
115. Lakhdar R, Al-Mallah MH, Lanfear DE. Safety and tolerability of angiotensin-converting enzyme inhibitor versus the combination of angiotensin-converting enzyme inhibitor and angiotensin receptor blocker in patients with left ventricular dysfunction: a systematic review and meta-analysis of randomized controlled trials. J Card Fail 2008;14: 181-8.
116. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334: 1349-55.
117. Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlledrelease metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERITHF Study Group. JAMA 2000;283:1295-302.
118. Packer M, Fowler MB, Roecker EB, et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002;106:2194-9.
119. Bristow MR, Gilbert EM, Abraham WT, et al. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996;94:2807-16.
120. Randomised, placebo-controlled trial of carvedilol in patients with congestive heart failure due to ischaemic heart disease. Australia/New Zealand Heart Failure Research Collaborative Group. Lancet 1997;349: 375-80.
121. A randomized trial of beta-blockade in heart failure. The Cardiac Insufficiency Bisoprolol Study (CIBIS). CIBIS Investigators and Committees. Circulation 1994;90:1765-73.
122. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-21.
123. Maric C, Zheng W, Walther T. Interactions between angiotensin II and atrial natriuretic peptide in renomedullary interstitial cells: the role of neutral endopeptidase. Nephron Physiol 2006;103:p149-56.
124. Cruden NL, Fox KA, Ludlam CA, Johnston NR, Newby DE. Neutral endopeptidase inhibition augments vascular actions of bradykinin in patients treated with angiotensin-converting enzyme inhibition. Hypertension 2004;44:913-8.
125. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371: 993-1004.
126. Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J 2005;26:967-74.
127. Fox K, Ford I, Steg PG, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 2008;372:817-21.
128. McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med 2009;150:784-94.
129. Komajda M, Hanon O, Hochadel M, et al. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur Heart J 2009;30:478-86.
130. Flannery G, Gehrig-Mills R, Billah B, Krum H. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta-blockers. Am J Cardiol 2008;101:865-9.
131. Komajda M, Follath F, Swedberg K, et al. The EuroHeart Failure Survey programmeea survey on the quality of care among patients with heart failure in Europe. Part 2: treatment. Eur Heart J 2003;24:464-74.
132. DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs 2007;67(suppl 2):15-24.
133. Colin P, Ghaleh B, Monnet X, et al. Contributions of heart rate and contractility to myocardial oxygen balance during exercise. Am J Physiol Heart Circ Physiol 2003;284:H676-82.
134. Fox K, Ford I, Steg PG, et al. Ivabradine for patients with stable coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:807-16.
135. Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875-85.
136. Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med 1986;314:1547-52.
137. Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991;325:303-10.
138. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049-57.
139. Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336: 525-33.
140. Hood WB Jr, Dans AL, Guyatt GH, Jaeschke R, McMurray JJ. Digitalis for treatment of heart failure in patients in sinus rhythm. Cochrane Database Syst Rev 2014:CD002901.
141. Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSIHF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1223-30.
142. Tatarczyk T, Engl J, Ciardi C, et al. Analysis of long-chain omega-3 fatty acid content in fish-oil supplements. Wien Klin Wochenschr 2007;119:417-22.
143. Villani AM, Crotty M, Cleland LG, et al. Fish oil administration in older adults: is there potential for adverse events? A systematic review of the literature. BMC Geriatr 2013;13:41.
144. Kris-Etherton PM, Hill AM. N-3 fatty acids: food or supplements? J Am Diet Assoc 2008;108:1125-30.
145. Kjekshus J, Apetrei E, Barrios V, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007;357:2248-61.
146. van der Harst P, Voors AA, van Gilst WH, Bohm M, van Veldhuisen DJ. Statins in the treatment of chronic heart failure: a systematic review. PLoS Med 2006;3:e333.
147. Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008;372:1231-9.
148. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60.
149. Massie BM, Collins JF, Ammon SE, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation 2009;119:1616-24.
150. Homma S, Thompson JL, Pullicino PM, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med 2012;366: 1859-69.
151. Hopper I, Skiba M, Krum H. Updated meta-analysis on antithrombotic therapy in patients with heart failure and sinus rhythm. Eur J Heart Fail 2013;15:69-78.
152. Kumar G, Goyal MK. Warfarin versus aspirin for prevention of stroke in heart failure: a meta-analysis of randomized controlled clinical trials. J Stroke Cerebrovasc Dis 2013;22:1279-87.
153. Solheim S, Seljeflot I, Lunde K, et al. Frequency of left ventricular thrombus in patients with anterior wall acute myocardial infarction treated with percutaneous coronary intervention and dual antiplatelet therapy. Am J Cardiol 2010;106:1197-200.
154. Nikolsky E, Mehran R, Dangas GD, et al. Outcomes of patients treated with triple antithrombotic therapy after primary percutaneous coronary intervention for ST-elevation myocardial infarction (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction [HORIZONS-AMI] trial). Am J Cardiol 2012;109:831-8.
155. Schwalm JD, Ahmad M, Salehian O, Eikelboom JW, Natarajan MK. Warfarin after anterior myocardial infarction in current era of dual antiplatelet therapy: a randomized feasibility trial. J Thromb Thrombolysis 2010;30:127-32.
156. Le May MR, Acharya S, Wells GA, et al. Prophylactic warfarin therapy after primary percutaneous coronary intervention for anterior STsegment elevation myocardial infarction. JACC Cardiovasc Interv 2015;8:155-62.
157. Udell JA, Wang JT, Gladstone DJ, Tu JV. Anticoagulation after anterior myocardial infarction and the risk of stroke. PLoS One 2010;5: e12150.
158. Page J, Henry D. Consumption of NSAIDs and the development of congestive heart failure in elderly patients: an underrecognized public health problem. Arch Intern Med 2000;160:777-84.
159. Heerdink ER, Leufkens HG, Herings RM, et al. NSAIDs associated with increased risk of congestive heart failure in elderly patients taking diuretics. Arch Intern Med 1998;158:1108-12.
160. Mamdani M, Juurlink DN, Lee DS, et al. Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study. Lancet 2004;363:1751-6.
161. Gislason GH, Rasmussen JN, Abildstrom SZ, et al. Increased mortality and cardiovascular morbidity associated with use of nonsteroidal antiinflammatory drugs in chronic heart failure. Arch Intern Med 2009;169:141-9.
162. Hudson M, Richard H, Pilote L. Differences in outcomes of patients with congestive heart failure prescribed celecoxib, rofecoxib, or nonsteroidal anti-inflammatory drugs: population based study. BMJ 2005;330:1370.
163. Feenstra J, Heerdink ER, Grobbee DE, Stricker BH. Association of nonsteroidal anti-inflammatory drugs with first occurrence of heart failure and with relapsing heart failure: the Rotterdam Study. Arch Intern Med 2002;162:265-70.
164. Elkayam U, Amin J, Mehra A, et al. A prospective, randomized, doubleblind, crossover study to compare the efficacy and safety of chronic nifedipine therapy with that of isosorbide dinitrate and their combination in the treatment of chronic congestive heart failure. Circulation 1990;82:1954-61.
165. Littler WA, Sheridan DJ. Placebo controlled trial of felodipine in patients with mild to moderate heart failure. UK Study Group. Br Heart J 1995;73:428-33.
166. Goldstein RE, Boccuzzi SJ, Cruess D, Nattel S. Diltiazem increases lateonset congestive heart failure in postinfarction patients with early reduction in ejection fraction. The Adverse Experience Committee; and the Multicenter Diltiazem Postinfarction Research Group. Circulation 1991;83:52-60.
167. Effect of verapamil on mortality and major events after acute myocardial infarction (the Danish Verapamil Infarction Trial IIeDAVIT II). Am J Cardiol 1990;66:779-85.
168. Packer M, O’Connor CM, Ghali JK, et al. Effect of amlodipine on morbidity and mortality in severe chronic heart failure. Prospective Randomized Amlodipine Survival Evaluation Study Group. N Engl J Med 1996;335:1107-14.
169. Packer M, Carson P, Elkayam U, et al. Effect of amlodipine on the survival of patients with severe chronic heart failure due to a nonischemic cardiomyopathy: results of the PRAISE-2 study (prospective randomized amlodipine survival evaluation 2). JACC Heart Fail 2013;1: 308-14.
170. Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet 2003;362:777-81.
171. Rogers JK, Pocock SJ, McMurray JJ, et al. Analysing recurrent hospitalizations in heart failure: a review of statistical methodology, with application to CHARM-Preserved. Eur J Heart Fail 2014;16:33-40.
172. Cleland JG, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 2006;27:2338-45.
173. Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 2008;359: 2456-67.
174. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383-92.
175. De Denus S, O’Meara E, Desai AS, et al. Spironolactone metabolites in TOPCAT: new insights into regional variations. N Engl J Med 2017;376:1690-2.
176. Bavishi C, Chatterjee S, Ather S, Patel D, Messerli FH. Beta-blockers in heart failure with preserved ejection fraction: a meta-analysis. Heart Fail Rev 2015;20:193-201.
177. Liu F, Chen Y, Feng X, et al. Effects of beta-blockers on heart failure with preserved ejection fraction: a meta-analysis. PLoS One 2014;9: e90555.
178. Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215-25.
179. Yamamoto K, Origasa H, Hori M, J-DHF Investigators. Effects of carvedilol on heart failure with preserved ejection fraction: the Japanese Diastolic Heart Failure Study (J-DHF). Eur J Heart Fail 2013;15: 110-8.
180. van Veldhuisen DJ, Cohen-Solal A, Bohm M, et al. Beta-blockade with nebivolol in elderly heart failure patients with impaired and preserved left ventricular ejection fraction: data From SENIORS (Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure). J Am Coll Cardiol 2009;53:2150-8.
181. Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015;373: 2314-24.
182. Arnold JM, Liu P, Demers C, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure 2006: diagnosis and management. Can J Cardiol 2006;22:23-45.
183. Howlett JG, McKelvie RS, Arnold JM, et al. Canadian Cardiovascular Society Consensus Conference guidelines on heart failure, update 2009: diagnosis and management of right-sided heart failure, myocarditis, device therapy and recent important clinical trials. Can J Cardiol 2009;25:85-105.
184. McKelvie RS, Moe GW, Ezekowitz JA, et al. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can J Cardiol 2013;29:168-81.
185. Connolly SJ, Gent M, Roberts RS, et al. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 2000;101: 1297-302.
186. Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 1997;337:1576-83.
187. Kuck KH, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH). Circulation 2000;102:748-54.
188. Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J 2000;21:2071-8.
189. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005;352:225-37.
190. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002;346:877-83.
191. Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med 2004;351:2481-8.
192. Steinbeck G, Andresen D, Seidl K, et al. Defibrillator implantation early after myocardial infarction. N Engl J Med 2009;361:1427-36.
193. Ezekowitz JA, Rowe BH, Dryden DM, et al. Systematic review: implantable cardioverter defibrillators for adults with left ventricular systolic dysfunction. Ann Intern Med 2007;147:251-62.
194. Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 2004;350:2151-8.
195. Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med 2016;375:1221-30.
196. Golwala H, Bajaj NS, Arora G, Arora P. Implantable cardioverterdefibrillator for nonischemic cardiomyopathy: an updated meta-analysis. Circulation 2017;135:201-3.
197. Bennett M, Parkash R, Nery P, et al. Canadian Cardiovascular Society/ Canadian Heart Rhythm Society 2016 implantable cardioverterdefibrillator guidelines. Can J Cardiol 2017;33:174-88.
198. Exner DV, Birnie DH, Moe G, et al. Canadian Cardiovascular Society guidelines on the use of cardiac resynchronization therapy: evidence and patient selection. Can J Cardiol 2013;29:182-95.
199. Bigger JT Jr, Whang W, Rottman JN, et al. Mechanisms of death in the CABG Patch trial: a randomized trial of implantable cardiac defibrillator prophylaxis in patients at high risk of death after coronary artery bypass graft surgery. Circulation 1999;99:1416-21.
200. Goldenberg I, Moss AJ, McNitt S, et al. Time dependence of defibrillator benefit after coronary revascularization in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol 2006;47:1811-7.
201. Barsheshet A, Goldenberg I, Moss AJ, et al. Effect of elapsed time from coronary revascularization to implantation of a cardioverter defibrillator on long-term survival in the MADIT-II trial. J Cardiovasc Electrophysiol 2011;22:1237-42.
202. Bax JJ, Schinkel AF, Boersma E, et al. Extensive left ventricular remodeling does not allow viable myocardium to improve in left ventricular ejection fraction after revascularization and is associated with worse long-term prognosis. Circulation 2004;110:II18-22.
203. Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350:2140-50.
204. Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539-49.
205. Tang AS, Wells GA, Talajic M, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med 2010;363:2385-95.
206. Leon AR, Abraham WT, Curtis AB, et al. Safety of transvenous cardiac resynchronization system implantation in patients with chronic heart failure: combined results of over 2,000 patients from a multicenter study program. J Am Coll Cardiol 2005;46:2348-56.
207. Ailawadi G, Lapar DJ, Swenson BR, et al. Surgically placed left ventricular leads provide similar outcomes to percutaneous leads in patients with failed coronary sinus lead placement. Heart Rhythm 2010;7: 619-25.
208. Penicka M, Bartunek J, Lang O, et al. Severe left ventricular dyssynchrony is associated with poor prognosis in patients with moderate systolic heart failure undergoing coronary artery bypass grafting. J Am Coll Cardiol 2007;50:1315-23.
209. Pokushalov E, Romanov A, Prohorova D, et al. Coronary artery bypass grafting with concomitant cardiac resynchronisation therapy in patients with ischaemic heart failure and left ventricular dyssynchrony. Eur J Cardiothorac Surg 2010;38:773-80.
210. Healey JS, Merchant R, Simpson C, et al. Canadian Cardiovascular Society/Canadian Anesthesiologists’ Society/Canadian Heart Rhythm Society joint position statement on the perioperative management of patients with implanted pacemakers, defibrillators, and neurostimulating devices. Can J Cardiol 2012;28:141-51.
211. Gersh BJ, Maron BJ, Bonow RO, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2011;58:e212-60.
212. Authors/Task Force members, Elliott PM, Anastasakis A, et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733-79.
213. O’Mahony C, Tome-Esteban M, Lambiase PD, et al. A validation study of the 2003 American College of Cardiology/European Society of Cardiology and 2011 American College of Cardiology Foundation/ American Heart Association risk stratification and treatment algorithms for sudden cardiac death in patients with hypertrophic cardiomyopathy. Heart 2013;99:534-41.
214. O’Mahony C, Jichi F, Pavlou M, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014;35:2010-20.
215. Maron BJ, Casey SA, Chan RH, et al. Independent assessment of the European Society of Cardiology sudden death risk model for hypertrophic cardiomyopathy. Am J Cardiol 2015;116:757-64.
216. Vriesendorp PA, Schinkel AF, Liebregts M, et al. Validation of the 2014 European Society of Cardiology guidelines risk prediction model for the primary prevention of sudden cardiac death in hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol 2015;8:829-35.
217. Al-Majed NS, McAlister FA, Bakal JA, Ezekowitz JA. Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann Intern Med 2011;154:401-12.
218. Wells G, Parkash R, Healey JS, et al. Cardiac resynchronization therapy: a meta-analysis of randomized controlled trials. CMAJ 2011;183:421-9.
219. Chen S, Ling Z, Kiuchi MG, Yin Y, Krucoff MW. The efficacy and safety of cardiac resynchronization therapy combined with implantable cardioverter defibrillator for heart failure: a meta-analysis of 5674 patients. Europace 2013;15:992-1001.
220. Cleland JG, Abraham WT, Linde C, et al. An individual patient metaanalysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J 2013;34:3547-56.
221. Masoudi FA, Mi X, Curtis LH, et al. Comparative effectiveness of cardiac resynchronization therapy with an implantable cardioverterdefibrillator versus defibrillator therapy alone: a cohort study. Ann Intern Med 2014;160:603-11.
222. Woods B, Hawkins N, Mealing S, et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart 2015;101:1800-6.
223. Goldenberg I, Kutyifa V, Klein HU, et al. Survival with cardiacresynchronization therapy in mild heart failure. N Engl J Med 2014;370:1694-701.
224. Sipahi I, Carrigan TP, Rowland DY, Stambler BS, Fang JC. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med 2011;171:1454-62.
225. Bryant AR, Wilton SB, Lai MP, Exner DV. Association between QRS duration and outcome with cardiac resynchronization therapy: a systematic review and meta-analysis. J Electrocardiol 2013;46:147-55.
226. Birnie DH, Ha A, Higginson L, et al. Impact of QRS morphology and duration on outcomes after cardiac resynchronization therapy: results from the Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT). Circ Heart Fail 2013;6:1190-8.
227. Cunnington C, Kwok CS, Satchithananda DK, et al. Cardiac resynchronisation therapy is not associated with a reduction in mortality or heart failure hospitalisation in patients with non-left bundle branch block QRS morphology: meta-analysis of randomised controlled trials. Heart 2015;101:1456-62.
228. Healey JS, Hohnloser SH, Exner DV, et al. Cardiac resynchronization therapy in patients with permanent atrial fibrillation: results from the Resynchronization for Ambulatory Heart Failure Trial (RAFT). Circ Heart Fail 2012;5:566-70.
229. Hayes DL, Boehmer JP, Day JD, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm 2011;8:1469-75.
230. Yin J, Hu H, Wang Y, et al. Effects of atrioventricular nodal ablation on permanent atrial fibrillation patients with cardiac resynchronization therapy: a systematic review and meta-analysis. Clin Cardiol 2014;37: 707-15.
231. Tops LF, Schalij MJ, Bax JJ. The effects of right ventricular apical pacing on ventricular function and dyssynchrony implications for therapy. J Am Coll Cardiol 2009;54:764-76.
232. Dilaveris P, Pantazis A, Giannopoulos G, et al. Upgrade to biventricular pacing in patients with pacing-induced heart failure: can resynchronization do the trick? Europace 2006;8:352-7.
233. Gierula J, Cubbon RM, Jamil HA, et al. Cardiac resynchronization therapy in pacemaker-dependent patients with left ventricular dysfunction. Europace 2013;15:1609-14.
234. Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med 2013;368:1585-93.
235. Ruschitzka F, Abraham WT, Singh JP, et al. Cardiac-resynchronization therapy in heart failure with a narrow QRS complex. N Engl J Med 2013;369:1395-405.
236. Thibault B, Harel F, Ducharme A, et al. Cardiac resynchronization therapy in patients with heart failure and a QRS complex <120 milliseconds: the Evaluation of Resynchronization Therapy for Heart Failure (LESSER-EARTH) trial. Circulation 2013;127:873-81.
237. Muto C, Solimene F, Gallo P, et al. A randomized study of cardiac resynchronization therapy defibrillator versus dual-chamber implantable cardioverter-defibrillator in ischemic cardiomyopathy with narrow QRS: the NARROW-CRT study. Circ Arrhythm Electrophysiol 2013;6: 538-45.
238. Wang G, Zhao Z, Zhao S, et al. Effect of cardiac resynchronization therapy on patients with heart failure and narrow QRS complexes: a meta-analysis of five randomized controlled trials. J Interv Card Electrophysiol 2015;44:71-9.
239. Shah RM, Patel D, Molnar J, Ellenbogen KA, Koneru JN. Cardiacresynchronization therapy in patients with systolic heart failure and QRS interval ≤130 ms: insights from a meta-analysis. Europace 2015;17:267-73.
240. Anand J, Singh SK, Antoun DG, et al. Durable mechanical circulatory support versus organ transplantation: past, present, and future. Biomed Res Int 2015;2015:849571.
241. Boehmer JP, Popjes E. Cardiac failure: mechanical support strategies. Crit Care Med 2006;34:S268-77.
242. Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant 2009;28:535-41.
243. Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014:CD003331.
244. Taylor RS, Davies EJ, Dalal HM, et al. Effects of exercise training for heart failure with preserved ejection fraction: a systematic review and meta-analysis of comparative studies. Int J Cardiol 2012;162:6-13.
245. Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med 2004;116:693-706.
246. O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439-50.
247. McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev 2008;13:3-11.
248. Davies EJ, Moxham T, Rees K, et al. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail 2010;12:706-15.
249. Ades PA, Keteyian SJ, Balady GJ, et al. Cardiac rehabilitation exercise and self-care for chronic heart failure. JACC Heart Fail 2013;1:540-7.
250. Ismail H, McFarlane JR, Nojoumian AH, Dieberg G, Smart NA. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: a systematic review and meta-analysis. JACC Heart Fail 2013;1:514-22.
251. Flynn KE, Pina IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1451-9.
252. Warburton DE, Bredin SS. Reflections on physical activity and health: what should we recommend? Can J Cardiol 2016;32:495-504.
253. Piepoli MF, Conraads V, Corra U, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail 2011;13:347-57.
254. Edelmann F, Gelbrich G, Dungen HD, et al. Exercise training improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction: results of the Ex-DHF (Exercise training in Diastolic Heart Failure) pilot study. J Am Coll Cardiol 2011;58:1780-91.
255. Nolte K, Herrmann-Lingen C, Wachter R, et al. Effects of exercise training on different quality of life dimensions in heart failure with preserved ejection fraction: the Ex-DHF-P trial. Eur J Prev Cardiol 2015;22:582-93.
256. Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol 2013;62:584-92.
257. Isaksen K, Morken IM, Munk PS, Larsen AI. Exercise training and cardiac rehabilitation in patients with implantable cardioverter defibrillators: a review of current literature focusing on safety, effects of exercise training, and the psychological impact of programme participation. Eur J Prev Cardiol 2012;19:804-12.
258. Vanhees L, Kornaat M, Defoor J, et al. Effect of exercise training in patients with an implantable cardioverter defibrillator. Eur Heart J 2004;25:1120-6.
259. Doukky R, Avery E, Mangla A, et al. Impact of Dietary Sodium Restriction On Heart Failure Outcomes. JACC Heart Fail 2016;4:24-35.
260. Colin-Ramirez E, McAlister FA, Zheng Y, et al. The long-term effects of dietary sodium restriction on clinical outcomes in patients with heart failure. The SODIUM-HF (Study of Dietary Intervention Under 100 mmol in Heart Failure): a pilot study. Am Heart J 2015;169:274-281.e1.
261. McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. J Am Soc Nephrol 2013;24: 2096-103.
262. Colin RE, Castillo ML, Orea TA, Montaño HP, Dorantes GJ. Impact of a sodium and fluid restricted diet on clinical status in heart failure patients [in Spanish]. Rev Chil Nutr 2010;37:427-37.
263. Arcand J, Ivanov J, Sasson A, et al. A high-sodium diet is associated with acute decompensated heart failure in ambulatory heart failure patients: a prospective follow-up study. Am J Clin Nutr 2011;93:332-7.
264. Gupta D, Georgiopoulou VV, Kalogeropoulos AP, et al. Dietary sodium intake in heart failure. Circulation 2012;126:479-85.
265. Aliti GB, Rabelo ER, Clausell N, et al. Aggressive fluid and sodium restriction in acute decompensated heart failure: a randomized clinical trial. JAMA Intern Med 2013;173:1058-64.
266. Holst M, Stromberg A, Lindholm M, Willenheimer R. Liberal versus restricted fluid prescription in stabilised patients with chronic heart failure: result of a randomised cross-over study of the effects on healthrelated quality of life, physical capacity, thirst and morbidity. Scand Cardiovasc J 2008;42:316-22.
267. Travers B, O’Loughlin C, Murphy NF, et al. Fluid restriction in the management of decompensated heart failure: no impact on time to clinical stability. J Card Fail 2007;13:128-32.
268. Nicolas JM, Fernandez-Sola J, Estruch R, et al. The effect of controlled drinking in alcoholic cardiomyopathy. Ann Intern Med 2002;136: 192-200.
269. Macle L, Cairns J, Leblanc K, et al. 2016 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol 2016;32:1170-85.
270. Chiang CE, Naditch-Brule L, Murin J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 2012;5:632-9.
271. van Deursen VM, Urso R, Laroche C, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail 2014;16:103-11.
272. Maisel WH, Stevenson LW. Atrial fibrillation in heart failure: epidemiology, pathophysiology, and rationale for therapy. Am J Cardiol 2003;91:2D-8D.
273. Mamas MA, Caldwell JC, Chacko S, et al. A meta-analysis of the prognostic significance of atrial fibrillation in chronic heart failure. Eur J Heart Fail 2009;11:676-83.
274. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920-5.
275. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983-8.
276. Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med 2008;358:2667-77.
277. Canadian Cardiovascular Society Heart Failure Management Primary Panel, Moe GW, Ezekowitz JA, et al. The 2013 Canadian Cardiovascular Society heart failure management guidelines update: focus on rehabilitation and exercise and surgical coronary revascularization. Can J Cardiol 2014;30:249-63.
278. Fox KF, Cowie MR, Wood DA, et al. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J 2001;22: 228-36.
279. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008;101:1016-22.
280. Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in collaboration with the American Academy of Family Physicians, Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2011;57:e215-367.
281. Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283-91.
282. Grace SL, Chessex C, Arthur H, et al. Systematizing inpatient referral to cardiac rehabilitation 2010: Canadian Association of Cardiac Rehabilitation and Canadian Cardiovascular Society joint position paper endorsed by the Cardiac Care Network of Ontario. Can J Cardiol 2011;27:192-9.
283. Alderman EL, Bourassa MG, Cohen LS, et al. Ten-year follow-up of survival and myocardial infarction in the randomized Coronary Artery Surgery Study. Circulation 1990;82:1629-46.
284. Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group. Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable angina. N Engl J Med 1984;311:1333-9.
285. Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med 1988;319:332-7.
286. Yusuf S, Zucker D, Peduzzi P, et al. Effect of coronary artery bypass graft surgery on survival: overview of 10-year results from randomised trials by the Coronary Artery Bypass Graft Surgery Trialists Collaboration. Lancet 1994;344:563-70.
287. Velazquez EJ, Lee KL, O’Connor CM, et al. The rationale and design of the Surgical Treatment for Ischemic Heart Failure (STICH) trial. J Thorac Cardiovasc Surg 2007;134:1540-7.
288. Kunadian V, Zaman A, Qiu W. Revascularization among patients with severe left ventricular dysfunction: a meta-analysis of observational studies. Eur J Heart Fail 2011;13:773-84.
289. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 2009;53:e1-90.
290. Gavazzi A, Berzuini C, Campana C, et al. Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant 1997;16:774-85.
291. Meyer P, Filippatos GS, Ahmed MI, et al. Effects of right ventricular ejection fraction on outcomes in chronic systolic heart failure. Circulation 2010;121:252-8.
292. Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001;37:183-8.
293. Gulati A, Ismail TF, Jabbour A, et al. The prevalence and prognostic significance of right ventricular systolic dysfunction in nonischemic dilated cardiomyopathy. Circulation 2013;128:1623-33.
294. Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): Consensus Panel report. J Cardiovasc Magn Reson 2004;6:727-65.
295. Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34-41.
296. Beck-da-Silva L, de Bold A, Davies R, et al. Effect of bisoprolol on right ventricular function and brain natriuretic peptide in patients with heart failure. Congest Heart Fail 2004;10:127-32.
297. Quaife RA, Christian PE, Gilbert EM, et al. Effects of carvedilol on right ventricular function in chronic heart failure. Am J Cardiol 1998;81:247-50.
298. Morrell NW, Higham MA, Phillips PG, et al. Pilot study of losartan for pulmonary hypertension in chronic obstructive pulmonary disease. Respir Res 2005;6:88.
299. Dore A, Houde C, Chan KL, et al. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation 2005;112: 2411-6.
300. McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573-619.
301. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation 2008;118:2395-451.
302. Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016;37:67-119.
303. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST guideline and expert panel report. Chest 2014;146:449-75.
304. Jain A, Shehata ML, Stuber M, et al. Prevalence of left ventricular regional dysfunction in arrhythmogenic right ventricular dysplasia: a tagged MRI study. Circ Cardiovasc Imaging 2010;3:290-7.
305. Basso C, Corrado D, Marcus FI, Nava A, Thiene G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 2009;373:1289-300.
306. Sen-Chowdhry S, Morgan RD, Chambers JC, McKenna WJ. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu Rev Med 2010;61:233-53.
307. Bauce B, Frigo G, Marcus FI, et al. Comparison of clinical features of arrhythmogenic right ventricular cardiomyopathy in men versus women. Am J Cardiol 2008;102:1252-7.
308. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533-41.
309. Borgquist R, Haugaa KH, Gilljam T, et al. The diagnostic performance of imaging methods in ARVC using the 2010 Task Force criteria. Eur Heart J Cardiovasc Imaging 2014;15:1219-25.
310. Sarvari SI, Haugaa KH, Anfinsen OG, et al. Right ventricular mechanical dispersion is related to malignant arrhythmias: a study of patients with arrhythmogenic right ventricular cardiomyopathy and subclinical right ventricular dysfunction. Eur Heart J 2011;32:1089-96.
311. te Riele AS, Tandri H, Bluemke DA. Arrhythmogenic right ventricular cardiomyopathy (ARVC): cardiovascular magnetic resonance update. J Cardiovasc Magn Reson 2014;16:50.
312. Marcus GM, Glidden DV, Polonsky B, et al. Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol 2009;54: 609-15.
313. Reiter MJ, Reiffel JA. Importance of beta blockade in the therapy of serious ventricular arrhythmias. Am J Cardiol 1998;82:9I-19I.
314. Corrado D, Calkins H, Link MS, et al. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation 2010;122:1144-52.
315. European Heart Rhythm Association; Heart Rhythm Society, Zipes DP, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death). J Am Coll Cardiol 2006;48:e247-346.
316. Leclercq JF, Potenza S, Maison-Blanche P, Chastang C, Coumel P. Determinants of spontaneous occurrence of sustained monomorphic ventricular tachycardia in right ventricular dysplasia. J Am Coll Cardiol 1996;28:720-4.
317. Furlanello F, Bertoldi A, Dallago M, et al. Cardiac arrest and sudden death in competitive athletes with arrhythmogenic right ventricular dysplasia. Pacing Clin Electrophysiol 1998;21:331-5.
318. Burke AP, Robinson S, Radentz S, Smialek J, Virmani R. Sudden death in right ventricular dysplasia with minimal gross abnormalities. J Forensic Sci 1999;44:438-43.
319. Chen RF, Lai CP. Clinical characteristics and treatment of constrictive pericarditis in Taiwan. Circ J 2005;69:458-60.
320. Adler Y, Charron P, Imazio M, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015;36:2921-64.
321. Ling LH, Oh JK, Breen JF, et al. Calcific constrictive pericarditis: is it still with us? Ann Intern Med 2000;132:444-50.
322. Welch TD, Ling LH, Espinosa RE, et al. Echocardiographic diagnosis of constrictive pericarditis: Mayo Clinic criteria. Circ Cardiovasc Imaging 2014;7:526-34.
323. Oh JK, Hatle LK, Seward JB, et al. Diagnostic role of Doppler echocardiography in constrictive pericarditis. J Am Coll Cardiol 1994;23: 154-62.
324. Klein AL, Abbara S, Agler DA, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr 2013;26:965-1012. e15.
325. Kusunose K, Dahiya A, Popovic ZB, et al. Biventricular mechanics in constrictive pericarditis comparison with restrictive cardiomyopathy and impact of pericardiectomy. Circ Cardiovasc Imaging 2013;6:399-406.
326. Nishimura RA, Carabello BA. Hemodynamics in the cardiac catheterization laboratory of the 21st century. Circulation 2012;125:2138-50.
327. Haley JH, Tajik AJ, Danielson GK, et al. Transient constrictive pericarditis: causes and natural history. J Am Coll Cardiol 2004;43:271-5.
328. Yetkin U, Kestelli M, Yilik L, et al. Recent surgical experience in chronic constrictive pericarditis. Tex Heart Inst J 2003;30:27-30.
329. Tirilomis T, Unverdorben S, von der Emde J. Pericardectomy for chronic constrictive pericarditis: risks and outcome. Eur J Cardiothorac Surg 1994;8:487-92.
330. World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System (VMNIS). Geneva: World Health Organization, 2011.
331. Triposkiadis F, Giamouzis G, Parissis J, et al. Reframing the association and significance of co-morbidities in heart failure. Eur J Heart Fail 2016;18:744-58.
332. Cleland JG, Zhang J, Pellicori P, et al. Prevalence and outcomes of anemia and hematinic deficiencies in patients with chronic heart failure. JAMA Cardiol 2016;1:539-47.
333. O’Meara E, Rouleau JL, White M, et al. Heart failure with anemia: novel findings on the roles of renal disease, interleukins, and specific left ventricular remodeling processes. Circ Heart Fail 2014;7:773-81.
334. O’Meara E, Clayton T, McEntegart MB, et al. Clinical correlates and consequences of anemia in a broad spectrum of patients with heart failure: results of the Candesartan in Heart Failure: Assessment of Reduction in Mortality and Morbidity (CHARM) Program. Circulation 2006;113:986-94.
335. Anand IS, Kuskowski MA, Rector TS, et al. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT. Circulation 2005;112:1121-7.
336. Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol 2002;39: 1780-6.
337. Ebner N, Jankowska EA, Ponikowski P, et al. The impact of iron deficiency and anaemia on exercise capacity and outcomes in patients with chronic heart failure. Results from the Studies Investigating Co-morbidities Aggravating Heart Failure. Int J Cardiol 2016;205:6-12.
338. Felker GM, Shaw LK, Stough WG, O’Connor CM. Anemia in patients with heart failure and preserved systolic function. Am Heart J 2006;151:457-62.
339. Hillege HL, Nitsch D, Pfeffer MA, et al. Renal function as a predictor of outcome in a broad spectrum of patients with heart failure. Circulation 2006;113:671-8.
340. McClellan WM, Flanders WD, Langston RD, Jurkovitz C, Presley R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: a population-based study. J Am Soc Nephrol 2002;13: 1928-36.
341. Davis JD, Olsen MA, Bommarito K, et al. All-payer analysis of heart failure hospitalization 30-day readmission: comorbidities matter. Am J Med 2017;130:93.e9-28.
342. Nordyke RJ, Kim JJ, Goldberg GA, et al. Impact of anemia on hospitalization time, charges, and mortality in patients with heart failure. Value Health 2004;7:464-71.
343. Lund LH, Donal E, Oger E, et al. Association between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur J Heart Fail 2014;16:992-1001.
344. Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. J Am Coll Cardiol 2012;59:998-1005.
345. Klip IT, Comin-Colet J, Voors AA, et al. Iron deficiency in chronic heart failure: an international pooled analysis. Am Heart J 2013;165: 575-582.e3.
346. Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency: an ominous sign in patients with systolic chronic heart failure. Eur Heart J 2010;31:1872-80.
347. Silverberg DS, Wexler D, Schwartz D. Is correction of iron deficiency a new addition to the treatment of the heart failure? Int J Mol Sci 2015;16:14056-74.
348. Cohen-Solal A, Leclercq C, Deray G, et al. Iron deficiency: an emerging therapeutic target in heart failure. Heart 2014;100:1414-20.
349. O’Meara E, de Denus S. Management of anemia and iron deficiency in heart failure. Curr Treat Options Cardiovasc Med 2010;12:532-48.
350. Dalimunthe NN, Lubis AR. Usefulness of reticulocyte hemoglobin equivalent in management of regular hemodialysis patients with iron deficiency anemia. Rom J Intern Med 2016;54:31-6.
351. Jankowska EA, von Haehling S, Anker SD, Macdougall IC, Ponikowski P. Iron deficiency and heart failure: diagnostic dilemmas and therapeutic perspectives. Eur Heart J 2013;34:816-29.
352. Avni T, Leibovici L, Gafter-Gvili A. Iron supplementation for the treatment of chronic heart failure and iron deficiency: systematic review and meta-analysis. Eur J Heart Fail 2012;14:423-9.
353. Qian C, Wei B, Ding J, Wu H, Wang Y. The efficacy and safety of iron supplementation in patients with heart failure and iron deficiency: a systematic review and meta-analysis. Can J Cardiol 2016;32:151-9.
354. Ponikowski P, van Veldhuisen DJ, Comin-Colet J, et al. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiencydagger. Eur Heart J 2015;36:657-68.
355. Anker SD, Comin Colet J, Filippatos G, et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009;361: 2436-48.
356. van Veldhuisen DJ, Ponikowski P, van der Meer P, et al. Effect of ferric carboxymaltose on exercise capacity in patients with chronic heart failure and iron deficiency. Circulation 2017;136(15):1374-83.
357. O’Meara E, de Denus S, Lepage S. Heart failure, iron deficiency, and supplementation: where do we stand? Can J Cardiol 2016;32:148-50.
358. Moe GW, Ezekowitz JA, O’Meara E, et al. The 2014 Canadian Cardiovascular Society heart failure management guidelines focus update: anemia, biomarkers, and recent therapeutic trial implications. Can J Cardiol 2015;31:3-16.
359. Lewis GD, Semigran MJ, Givertz MM, et al. Oral iron therapy for heart failure with reduced ejection fraction: design and rationale for oral iron repletion effects on oxygen uptake in heart failure. Circ Heart Fail 2016;9:e000345.
360. Ghali JK, Anand IS, Abraham WT, et al. Randomized double-blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation 2008;117:526-35.
361. Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med 2013;368: 1210-9.
362. Arora NP, Ghali JK. Anemia and iron deficiency in heart failure. Heart Fail Clin 2014;10:281-94.
363. Collister D, Komenda P, Hiebert B, et al. The effect of erythropoietinstimulating agents on health-related quality of life in anemia of chronic kidney disease: a systematic review and meta-analysis. Ann Intern Med 2016;164:472-8.
364. Margulies KB, Hernandez AF, Redfield MM, et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 2016;316: 500-8.
365. Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagonlike peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail 2017;19:69-77.
366. Smith GL, Lichtman JH, Bracken MB, et al. Renal impairment and outcomes in heart failure: systematic review and meta-analysis. J Am Coll Cardiol 2006;47:1987-96.
367. Harnett JD, Foley RN, Kent GM, et al. Congestive heart failure in dialysis patients: prevalence, incidence, prognosis and risk factors. Kidney Int 1995;47:884-90.
368. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976;16:31-41.
369. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1-266.
370. Bock JS, Gottlieb SS. Cardiorenal syndrome: new perspectives. Circulation 2010;121:2592-600.
371. Ronco C, McCullough P, Anker SD, et al. Cardio-renal syndromes: report from the consensus conference of the acute dialysis quality initiative. Eur Heart J 2010;31:703-11.
372. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009;53:589-96.
373. Damman K, van Deursen VM, Navis G, et al. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J Am Coll Cardiol 2009;53:582-8.
374. Braam B, Joles JA, Danishwar AH, Gaillard CA. Cardiorenal syndromeecurrent understanding and future perspectives. Nat Rev Nephrol 2014;10:48-55.
375. Palevsky PM. Renal replacement therapy I: indications and timing. Crit Care Clin 2005;21:347-56.
376. Cice G, Ferrara L, D’Andrea A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol 2003;41:1438-44.
377. Winkelmayer WC, Charytan DM, Levin R, Avorn J. Poor short-term survival and low use of cardiovascular medications in elderly dialysis patients after acute myocardial infarction. Am J Kidney Dis 2006;47: 301-8.
378. Zannad F, Kessler M, Lehert P, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int 2006;70:1318-24.
379. Suzuki H, Kanno Y, Sugahara S, et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis 2008;52: 501-6.
380. Cice G, Di Benedetto A, D’Isa S, et al. Effects of telmisartan added to Angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol 2010;56:1701-8.
381. Hussain S, Dreyfus DE, Marcus RJ, Biederman RW, McGill RL. Is spironolactone safe for dialysis patients? Nephrol Dial Transplant 2003;18:2364-8.
382. Saudan P, Mach F, Perneger T, et al. Safety of low-dose spironolactone administration in chronic haemodialysis patients. Nephrol Dial Transplant 2003;18:2359-63.
383. Gross E, Rothstein M, Dombek S, Juknis HI. Effect of spironolactone on blood pressure and the renin-angiotensin-aldosterone system in oligo-anuric hemodialysis patients. Am J Kidney Dis 2005;46:94-101.
384. Taheri S, Mortazavi M, Shahidi S, et al. Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi J Kidney Dis Transpl 2009;20:392-7.
385. Knoll G, Cockfield S, Blydt-Hansen T, et al. Canadian Society of Transplantation: consensus guidelines on eligibility for kidney transplantation. CMAJ 2005;173:S1-25.
386. Ferreira SR, Moises VA, Tavares A, Pacheco-Silva A. Cardiovascular effects of successful renal transplantation: a 1-year sequential study of left ventricular morphology and function, and 24-hour blood pressure profile. Transplantation 2002;74:1580-7.
387. Wali RK, Wang GS, Gottlieb SS, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol 2005;45: 1051-60.
388. Parfrey PS, Harnett JD, Foley RN, et al. Impact of renal transplantation on uremic cardiomyopathy. Transplantation 1995;60:908-14.
389. Fonarow GC, Corday E. ADHERE Scientific Advisory Committee. Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry. Heart Fail Rev 2004;9:179-85.
390. Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. Am J Cardiol 2006;97:1759-64.
391. Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J 2004;147:331-8.
392. Bart BA. Treatment of congestion in congestive heart failure: ultrafiltration is the only rational initial treatment of volume overload in decompensated heart failure. Circ Heart Fail 2009;2:499-504.
393. Bart BA, Boyle A, Bank AJ, et al. Ultrafiltration versus usual care for hospitalized patients with heart failure: the Relief for Acutely FluidOverloaded Patients With Decompensated Congestive Heart Failure (RAPID-CHF) trial. J Am Coll Cardiol 2005;46:2043-6.
394. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675-83.
395. Bart BA, Goldsmith SR, Lee KL, et al. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012;367: 2296-304.
396. Lyons OD, Bradley TD. Heart failure and sleep apnea. Can J Cardiol 2015;31:898-908.
397. Cormican LJ, Williams A. Sleep disordered breathing and its treatment in congestive heart failure. Heart 2005;91:1265-70.
398. Ferrier K, Campbell A, Yee B, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest 2005;128:2116-22.
399. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep 1999;22:667-89.
400. Sin DD, Logan AG, Fitzgerald FS, Liu PP, Bradley TD. Effects of continuous positive airway pressure on cardiovascular outcomes in heart failure patients with and without Cheyne-Stokes respiration. Circulation 2000;102:61-6.
401. Bradley TD, Logan AG, Kimoff RJ, et al. Continuous positive airway pressure for central sleep apnea and heart failure. N Engl J Med 2005;353:2025-33.
402. Teschler H, Dohring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med 2001;164:614-9.
403. Cowie MR, Woehrle H, Wegscheider K, et al. Adaptive servoventilation for central sleep apnea in systolic heart failure. N Engl J Med 2015;373:1095-105.
404. McCormack JP, Loewen P. Adding “value” to clinical practice guidelines. Can Fam Physician 2007;53:1326-7.
405. Adams KF Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J 2005;149:209-16.
406. Arnold JM, Howlett JG, Dorian P, et al. Canadian Cardiovascular Society consensus conference recommendations on heart failure update 2007: prevention, management during intercurrent illness or acute decompensation, and use of biomarkers. Can J Cardiol 2007;23:21-45.
407. Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003;41:1797-804.
408. Ghali JK, Kadakia S, Cooper R, Ferlinz J. Precipitating factors leading to decompensation of heart failure. Traits among urban blacks. Arch Intern Med 1988;148:2013-6.
409. Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart Failure Survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006;27:2725-36.
410. Van de Werf F, Ardissino D, Betriu A, et al. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J 2003;24:28-66.
411. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 2002;347:161-7.
412. Moe GW, Howlett J, Januzzi JL, Zowall H, Canadian Multicenter Improved Management of Patients With Congestive Heart Failure (IMPROVE-CHF) Study Investigators. N-terminal pro-B-type natriuretic peptide testing improves the management of patients with suspected acute heart failure: primary results of the Canadian prospective randomized multicenter IMPROVE-CHF study. Circulation 2007;115:3103-10.
413. Baggish AL, Siebert U, Lainchbury JG, et al. A validated clinical and biochemical score for the diagnosis of acute heart failure: the ProBNP Investigation of Dyspnea in the emergency department (PRIDE) Acute Heart Failure Score. Am Heart J 2006;151:48-54.
414. Steinhart B, Thorpe KE, Bayoumi AM, et al. Improving the diagnosis of acute heart failure using a validated prediction model. J Am Coll Cardiol 2009;54:1515-21.
415. Park JH, Balmain S, Berry C, Morton JJ, McMurray JJ. Potentially detrimental cardiovascular effects of oxygen in patients with chronic left ventricular systolic dysfunction. Heart 2010;96:533-8.
416. Sepehrvand N, Ezekowitz JA. Oxygen therapy in patients with acute heart failure: friend or foe? JACC Heart Fail 2016;4:783-90.
417. Gray A, Goodacre S, Seah M, Tilley S. Diuretic, opiate and nitrate use in severe acidotic acute cardiogenic pulmonary oedema: analysis from the 3CPO trial. QJM 2010;103:573-81.
418. Peacock WF, Hollander JE, Diercks DB, et al. Morphine and outcomes in acute decompensated heart failure: an ADHERE analysis. Emerg Med J 2008;25:205-9.
419. Iakobishvili Z, Cohen E, Garty M, et al. Use of intravenous morphine for acute decompensated heart failure in patients with and without acute coronary syndromes. Acute Card Care 2011;13:76-80.
420. Gray A, Goodacre S, Newby DE, et al. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med 2008;359:142-51.
421. Vital FM, Ladeira MT, Atallah AN. Non-invasive positive pressure ventilation (CPAP or bilevel NPPV) for cardiogenic pulmonary oedema. Cochrane Database Syst Rev 2013;5:CD005351.
422. Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011;364:797-805.
423. Brater DC. Diuretic therapy. N Engl J Med 1998;339:387-95.
424. Wilson JR, Reichek N, Dunkman WB, Goldberg S. Effect of diuresis on the performance of the failing left ventricle in man. Am J Med 1981;70:234-9.
425. Gardtman M, Waagstein L, Karlsson T, Herlitz J. Has an intensified treatment in the ambulance of patients with acute severe left heart failure improved the outcome? Eur J Emerg Med 2000;7:15-24.
426. Ducharme A, Doyon O, White M, Rouleau JL, Brophy JM. Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial. CMAJ 2005;173:40-5.
427. Kiyingi A, Field MJ, Pawsey CC, et al. Metolazone in treatment of severe refractory congestive cardiac failure. Lancet 1990;335:29-31.
428. van Vliet AA, Donker AJ, Nauta JJ, Verheugt FW. Spironolactone in congestive heart failure refractory to high-dose loop diuretic and lowdose angiotensin-converting enzyme inhibitor. Am J Cardiol 1993;71: 21A-8A.
429. Wu MY, Chang NC, Su CL, et al. Loop diuretic strategies in patients with acute decompensated heart failure: a meta-analysis of randomized controlled trials. J Crit Care 2014;29:2-9.
430. ter Maaten JM, Dunning AM, Valente MA, et al. Diuretic response in acute heart failure-an analysis from ASCEND-HF. Am Heart J 2015;170:313-21.
431. Valente MA, Voors AA, Damman K, et al. Diuretic response in acute heart failure: clinical characteristics and prognostic significance. Eur Heart J 2014;35:1284-93.
432. Alexander P, Alkhawam L, Curry J, et al. Lack of evidence for intravenous vasodilators in ED patients with acute heart failure: a systematic review. Am J Emerg Med 2015;33:133-41.
433. Cotter G, Metzkor E, Kaluski E, et al. Randomised trial of high-dose isosorbide dinitrate plus low-dose furosemide versus high-dose furosemide plus low-dose isosorbide dinitrate in severe pulmonary oedema. Lancet 1998;351:389-93.
434. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 2002;287:1531-40.
435. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32-43.
436. Mullens W, Abrahams Z, Francis GS, et al. Sodium nitroprusside for advanced low-output heart failure. J Am Coll Cardiol 2008;52:200-7.
437. Packer M, Holcomb R, Abraham WT, et al. Rationale for and design of the TRUE-AHF trial: the effects of ularitide on the short-term clinical course and long-term mortality of patients with acute heart failure. Eur J Heart Fail 2017;19:673-81.
438. Felker GM, Butler J, Collins SP, et al. Heart failure therapeutics on the basis of a biased ligand of the angiotensin-2 type 1 receptor. Rationale and design of the BLAST-AHF study (Biased Ligand of the Angiotensin Receptor Study in Acute Heart Failure). JACC Heart Fail 2015;3: 193-201.
439. Cleland JG, Freemantle N, Coletta AP, Clark AL. Clinical trials update from the American Heart Association: REPAIR-AMI, ASTAMI, JELIS, MEGA, REVIVE-II, SURVIVE, and PROACTIVE. Eur J Heart Fail 2006;8:105-10.
440. Cuffe MS, Califf RM, Adams KF Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA 2002;287:1541-7.
441. Felker GM, Benza RL, Chandler AB, et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol 2003;41:997-1003.
442. Packer M, Colucci W, Fisher L, et al. Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure. JACC Heart Fail 2013;1:103-11.
443. Teerlink JR, Felker GM, McMurray JJ, et al. Acute Treatment With Omecamtiv Mecarbil to Increase Contractility in Acute Heart Failure: the ATOMIC-AHF study. J Am Coll Cardiol 2016;67:1444-55.
444. Chen HH, Anstrom KJ, Givertz MM, et al. Low-dose dopamine or low-dose nesiritide in acute heart failure with renal dysfunction: the ROSE acute heart failure randomized trial. JAMA 2013;310:2533-43.
445. Triposkiadis FK, Butler J, Karayannis G, et al. Efficacy and safety of high dose versus low dose furosemide with or without dopamine infusion: the Dopamine in Acute Decompensated Heart Failure II (DAD-HF II) trial. Int J Cardiol 2014;172:115-21.
446. Ezekowitz JA, Bakal JA, Kaul P, Westerhout CM, Armstrong PW. Acute heart failure in the emergency department: short and long-term outcomes of elderly patients with heart failure. Eur J Heart Fail 2008;10:308-14.
447. Jondeau G, Neuder Y, Eicher JC, et al. B-CONVINCED: Beta-blocker CONtinuation Vs. INterruption in patients with Congestive heart failure hospitalizED for a decompensation episode. Eur Heart J 2009;30:2186-92.
448. Gheorghiade M, Gattis WA, O’Connor CM, et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA 2004;291:1963-71.
449. Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 2006;355:2099-112.
450. Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319-31.
451. Hauptman PJ, Burnett J, Gheorghiade M, et al. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail 2013;19:390-7.
452. Salah K, Pinto YM, Eurlings LW, et al. Serum potassium decline during hospitalization for acute decompensated heart failure is a predictor of 6- month mortality, independent of N-terminal pro-B-type natriuretic peptide levels: an individual patient data analysis. Am Heart J 2015;170: 531-542.e1.
453. Lee DS, Ezekowitz JA. Risk stratification in acute heart failure. Can J Cardiol 2014;30:312-9.
454. Collins SP, Jenkins CA, Harrell FE Jr, et al. Identification of emergency department patients with acute heart failure at low risk for 30-day adverse events: the STRATIFY decision tool. JACC Heart Fail 2015;3:737-47.
455. van Diepen S, Bakal JA, Lin M, et al. Variation in critical care unit admission rates and outcomes for patients with acute coronary syndromes or heart failure among high- and low-volume cardiac hospitals. J Am Heart Assoc 2015;4:e001708.
456. Cecconi M, Reynolds TE, Al-Subaie N, Rhodes A. Haemodynamic monitoring in acute heart failure. Heart Fail Rev 2007;12:105-11.
457. Binanay C, Califf RM, Hasselblad V, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA 2005;294:1625-33.
458. Shah MR, Hasselblad V, Stevenson LW, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA 2005;294:1664-70.
459. Pandey A, Khera R, Kumar N, et al. Use of pulmonary artery catheterization in US patients with heart failure, 2001-2012. JAMA Intern Med 2016;176:129-32.
460. Marik PE. Pulmonary artery catheterization and esophageal Doppler monitoring in the ICU. Chest 1999;116:1085-91.
461. Maron BJ, Ommen SR, Semsarian C, et al. Hypertrophic cardiomyopathy present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol 2014;64:83-99.
462. Spirito P, Chiarella F, Carratino L, et al. Clinical course and prognosis of hypertrophic cardiomyopathy in an outpatient population. N Engl J Med 1989;320:749-55.
463. Biagini E, Coccolo F, Ferlito M, et al. Dilated-hypokinetic evolution of hypertrophic cardiomyopathy: prevalence, incidence, risk factors, and prognostic implications in pediatric and adult patients. J Am Coll Cardiol 2005;46:1543-50.
464. Spirito P, Maron BJ, Bonow RO, Epstein SE. Occurrence and significance of progressive left ventricular wall thinning and relative cavity dilatation in hypertrophic cardiomyopathy. Am J Cardiol 1987;60: 123-9.
465. Child JS, Perloff JK, Bach PM, et al. Cardiac involvement in Friedreich’s ataxia: a clinical study of 75 patients. J Am Coll Cardiol 1986;7: 1370-8.
466. Sarkozy A, Conti E, Seripa D, et al. Correlation between PTPN11 gene mutations and congenital heart defects in Noonan and LEOPARD syndromes. J Med Genet 2003;40:704-8.
467. Maron BJ, Casey SA, Poliac LC, et al. Clinical course of hypertrophic cardiomyopathy in a regional United States cohort. JAMA 1999;281: 650-5.
468. Karamitsos TD, Francis JM, Myerson S, Selvanayagam JB, Neubauer S. The role of cardiovascular magnetic resonance imaging in heart failure. J Am Coll Cardiol 2009;54:1407-24.
469. Nagueh SF, Bachinski LL, Meyer D, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation 2001;104: 128-30.
470. Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med 2004;350:1320-7.
471. Artz G, Wynne J. Restrictive cardiomyopathy. Curr Treat Options Cardiovasc Med 2000;2:431-8.
472. Seward JB, Casaclang-Verzosa G. Infiltrative cardiovascular diseases: cardiomyopathies that look alike. J Am Coll Cardiol 2010;55:1769-79.
473. Kushwaha SS, Fallon JT, Fuster V. Restrictive cardiomyopathy. N Engl J Med 1997;336:267-76.
474. Mogensen J, Kubo T, Duque M, et al. Idiopathic restrictive cardiomyopathy is part of the clinical expression of cardiac troponin I mutations. J Clin Invest 2003;111:209-16.
475. Ammash NM, Seward JB, Bailey KR, Edwards WD, Tajik AJ. Clinical profile and outcome of idiopathic restrictive cardiomyopathy. Circulation 2000;101:2490-6.
476. Gertz MA, Lacy MQ, Dispenzieri A. Amyloidosis. Hematol Oncol Clin North Am 1999;13:1211-33, ix.
477. Reisinger J, Dubrey SW, Lavalley M, Skinner M, Falk RH. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J Am Coll Cardiol 1997;30:1046-51.
478. Rocken C, Peters B, Juenemann G, et al. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002;106:2091-7.
479. Clarke JT. Narrative review: Fabry disease. Ann Intern Med 2007;146: 425-33.
480. Dubrey SW, Bilazarian S, LaValley M, et al. Signal-averaged electrocardiography in patients with AL (primary) amyloidosis. Am Heart J 1997;134:994-1001.
481. Desai HV, Aronow WS, Peterson SJ, Frishman WH. Cardiac amyloidosis: approaches to diagnosis and management. Cardiol Rev 2010;18:1-11.
482. Falk RH, Alexander KM, Liao R, Dorbala S. AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol 2016;68:1323-41.
483. Lachmann HJ, Booth DR, Booth SE, et al. Misdiagnosis of hereditary amyloidosis as AL (primary) amyloidosis. N Engl J Med 2002;346: 1786-91.
484. Cacoub P, Axler O, De Zuttere D, et al. Amyloidosis and cardiac involvement. Ann Med Interne (Paris) 2000;151:611-7.
485. Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol 2007;50:2101-10.
486. Appleton CP, Hatle LK, Popp RL. Demonstration of restrictive ventricular physiology by Doppler echocardiography. J Am Coll Cardiol 1988;11:757-68.
487. Choi EY, Ha JW, Kim JM, et al. Incremental value of combining systolic mitral annular velocity and time difference between mitral inflow and diastolic mitral annular velocity to early diastolic annular velocity for differentiating constrictive pericarditis from restrictive cardiomyopathy. J Am Soc Echocardiogr 2007;20:738-43.
488. Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2000;2: 271-8.
489. Kim RJ, Fieno DS, Parrish TB, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation 1999;100:1992-2002.
490. Friedrich MG, Strohm O, Schulz-Menger J, et al. Contrast mediaenhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation 1998;97:1802-9.
491. Maceira AM, Joshi J, Prasad SK, et al. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation 2005;111:186-93.
492. Uemura A, Morimoto S, Hiramitsu S, et al. Histologic diagnostic rate of cardiac sarcoidosis: evaluation of endomyocardial biopsies. Am Heart J 1999;138:299-302.
493. Schulz-Menger J, Wassmuth R, Abdel-Aty H, et al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006;92:399-400.
494. Singh JA, Woodard PK, Davila-Roman VG, et al. Cardiac magnetic resonance imaging abnormalities in systemic lupus erythematosus: a preliminary report. Lupus 2005;14:137-44.
495. Petersen SE, Kardos A, Neubauer S. Subendocardial and papillary muscle involvement in a patient with Churg-Strauss syndrome, detected by contrast enhanced cardiovascular magnetic resonance. Heart 2005;91:e9.
496. Westwood MA, Anderson LJ, Firmin DN, et al. Interscanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J Magn Reson Imaging 2003;18:616-20.
497. Tanaka M, Hongo M, Kinoshita O, et al. Iodine-123 metaiodobenzylguanidine scintigraphic assessment of myocardial sympathetic innervation in patients with familial amyloid polyneuropathy. J Am Coll Cardiol 1997;29:168-74.
498. Canada S. Census of Population. 2006. Available at: http://www23. statcan.gc.ca/imdb/p2SV.pl?Function=getSurvey&SurvId=30216&InstaId= 30219&SDDS=3901. Accessed March 2, 2016.
499. Balsa AI, McGuire TG. Prejudice, clinical uncertainty and stereotyping as sources of health disparities. J Health Econ 2003;22:89-116.
500. Gerend MA, Pai M. Social determinants of black-white disparities in breast cancer mortality: a review. Cancer Epidemiol Biomarkers Prev 2008;17:2913-23.
501. Groman R, Ginsburg J. American College of Physicians. Racial and ethnic disparities in health care: a position paper of the American College of Physicians. Ann Intern Med 2004;141:226-32.
502. Woolf SH, Johnson RE, Fryer GE Jr, Rust G, Satcher D. The health impact of resolving racial disparities: an analysis of US mortality data. Am J Public Health 2008;98:S26-8.
503. Colvin M, Sweitzer NK, Albert NM, et al. Heart failure in nonCaucasians, women, and older adults: a white paper on special populations from the Heart Failure Society of America Guideline Committee. J Card Fail 2015;21:674-93.
504. Sanderson JE, Chan SK, Yip G, et al. Beta-blockade in heart failure: a comparison of carvedilol with metoprolol. J Am Coll Cardiol 1999;34: 1522-8.
505. Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937-52.
506. Tu JV, Chu A, Rezai MR, et al. The incidence of major cardiovascular events in immigrants to Ontario, Canada: the CANHEART Immigrant Study. Circulation 2015;132:1549-59.
507. Mentz RJ, Roessig L, Greenberg BH, et al. Heart failure clinical trials in East and Southeast Asia: understanding the importance and defining the next steps. JACC Heart Fail 2016;4:419-27.
508. Krauser DG, Chen AA, Tung R, et al. Neither race nor gender influences the usefulness of amino-terminal pro-brain natriuretic peptide testing in dyspneic subjects: a ProBNP Investigation of Dyspnea in the emergency department (PRIDE) substudy. J Card Fail 2006;12:452-7.
509. Ajayi AA, Balogun MO, Oyewo EA, Ladipo GO. Enalapril in African patients with congestive cardiac failure. Br J Clin Pharmacol 1989;27: 400-3.
510. Thorne S, MacGregor A, Nelson-Piercy C. Risks of contraception and pregnancy in heart disease. Heart 2006;92:1520-5.
511. Pijuan-Domènech A, Galian L, Goya M, et al. Cardiac complications during pregnancy are better predicted with the modified WHO risk score. Int J Cardiol 2015;195:149-54.
512. Regitz-Zagrosek V, Seeland U, Geibel-Zehender A, et al. Cardiovascular diseases in pregnancy. Dtsch Arztebl Int 2011;108:267-73.
513. Alonso-Gonzalez R, Swan L. Treating cardiac disease in pregnancy. Womens Health (Lond) 2014;10:79-88 [quiz: 89-90].
514. Herrey AS. Pregnancy in inherited and acquired cardiomyopathies. Best Pract Res Clin Obstet Gynaecol 2014;28:563-77.
515. Howlett JG, McKelvie RS, Costigan J, et al. The 2010 Canadian Cardiovascular Society guidelines for the diagnosis and management of heart failure update: heart failure in ethnic minority populations, heart failure and pregnancy, disease management, and quality improvement/ assurance programs. Can J Cardiol 2010;26:185-202.
516. European Society of Gynecology (ESG); Association for European Paediatric Cardiology (AEPC); German Society for Gender Medicine (DGesGM), et al. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2011;32:3147-97.
517. Cooper WO, Hernandez-Diaz S, Arbogast PG, et al. Major congenital malformations after first-trimester exposure to ACE inhibitors. N Engl J Med 2006;354:2443-51.
518. Schaefer C. Angiotensin II-receptor-antagonists: further evidence of fetotoxicity but not teratogenicity. Birth Defects Res A Clin Mol Teratol 2003;67:591-4.
519. Lydakis C, Lip GY, Beevers M, Beevers DG. Atenolol and fetal growth in pregnancies complicated by hypertension. Am J Hypertens 1999;12: 541-7.
520. Pearl W. Familial occurrence of peripartum cardiomyopathy. Am Heart J 1995;129:421-2.
521. Fett JD. Viral infection as a possible trigger for the development of peripartum cardiomyopathy. Int J Gynaecol Obstet 2007;97:149-50.
522. Sliwa K, Förster O, Libhaber E, et al. Peripartum cardiomyopathy: inflammatory markers as predictors of outcome in 100 prospectively studied patients. Eur Heart J 2006;27:441-6.
523. Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007;128:589-600.
524. Sliwa K, Hilfiker-Kleiner D, Petrie MC, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 2010;12:767-78.
525. Li W, Li H, Long Y. Clinical characteristics and long-term predictors of persistent left ventricular systolic dysfunction in peripartum cardiomyopathy. Can J Cardiol 2016;32:362-8.
526. Forster O, Hilfiker-Kleiner D, Ansari AA, et al. Reversal of IFN-gamma, oxLDL and prolactin serum levels correlate with clinical improvement in patients with peripartum cardiomyopathy. Eur J Heart Fail 2008;10: 861-8.
527. Desplantie O, Tremblay-Gravel M, Avram R, et al. The medical treatment of new-onset peripartum cardiomyopathy: a systematic review of prospective studies. Can J Cardiol 2015;31:1421-6.
528. Sliwa K, Blauwet L, Tibazarwa K, et al. Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-ofconcept pilot study. Circulation 2010;121:1465-73.
529. Haghikia A, Podewski E, Berliner D, et al. Rationale and design of a randomized, controlled multicentre clinical trial to evaluate the effect of bromocriptine on left ventricular function in women with peripartum cardiomyopathy. Clin Res Cardiol 2015;104:911-7.
530. Ballo P, Betti I, Mangialavori G, et al. Peripartum cardiomyopathy presenting with predominant left ventricular diastolic dysfunction: efficacy of bromocriptine. Case Rep Med 2012;2012:476903.
531. Elkayam U. Clinical characteristics of peripartum cardiomyopathy in the United States: diagnosis, prognosis, and management. J Am Coll Cardiol 2011;58:659-70.
532. McNamara DM, Elkayam U, Alharethi R, et al. Clinical outcomes for peripartum cardiomyopathy in North America: results of the IPAC study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 2015;66:905-14.
533. Goland S, Modi K, Bitar F, et al. Clinical profile and predictors of complications in peripartum cardiomyopathy. J Card Fail 2009;15: 645-50.
534. Elkayam U, Tummala PP, Rao K, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med 2001;344:1567-71.
535. Fett JD, Fristoe KL, Welsh SN. Risk of heart failure relapse in subsequent pregnancy among peripartum cardiomyopathy mothers. Int J Gynaecol Obstet 2010;109:34-6.
536. Elkayam U. Risk of subsequent pregnancy in women with a history of peripartum cardiomyopathy. J Am Coll Cardiol 2014;64:1629-36.
537. Aretz HT. Myocarditis: the Dallas criteria. Hum Pathol 1987;18: 619-24.
538. Baughman KL. Diagnosis of myocarditis: death of Dallas criteria. Circulation 2006;113:593-5.
539. Mason JW, O’Connell JB, Herskowitz A, et al. A clinical trial of immunosuppressive therapy for myocarditis. The Myocarditis Treatment Trial Investigators. N Engl J Med 1995;333:269-75.
540. Felker GM, Hu W, Hare JM, et al. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore) 1999;78:270-83.
541. Dec GW Jr, Palacios IF, Fallon JT, et al. Active myocarditis in the spectrum of acute dilated cardiomyopathies. Clinical features, histologic correlates, and clinical outcome. N Engl J Med 1985;312:885-90.
542. D’Ambrosio A, Patti G, Manzoli A, et al. The fate of acute myocarditis between spontaneous improvement and evolution to dilated cardiomyopathy: a review. Heart 2001;85:499-504.
543. Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation 1997;95:163-8.
544. Pinamonti B, Alberti E, Cigalotto A, et al. Echocardiographic findings in myocarditis. Am J Cardiol 1988;62:285-91.
545. Radunski UK, Lund GK, Stehning C, et al. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging 2014;7:667-75.
546. Zagrosek A, Abdel-Aty H, Boye P, et al. Cardiac magnetic resonance monitors reversible and irreversible myocardial injury in myocarditis. JACC Cardiovasc Imaging 2009;2:131-8.
547. Bohnen S, Radunski UK, Lund GK, et al. Performance of t1 and t2 mapping cardiovascular magnetic resonance to detect active myocarditis in patients with recent-onset heart failure. Circ Cardiovasc Imaging 2015;8:e003073.
548. Bonner F, Spieker M, Haberkorn S, et al. Myocardial T2 mapping increases noninvasive diagnostic accuracy for biopsy-proven myocarditis. JACC Cardiovasc Imaging 2016;9:1467-9.
549. Leone O, Veinot JP, Angelini A, et al. 2011 Consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc Pathol 2012;21:245-74.
550. Caforio AL, Calabrese F, Angelini A, et al. A prospective study of biopsy-proven myocarditis: prognostic relevance of clinical and aetiopathogenetic features at diagnosis. Eur Heart J 2007;28:1326-33.
551. Caforio AL, Pankuweit S, Arbustini E, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J 2013;34: 2636-48, 48a-48d.
552. Frustaci A, Russo MA, Chimenti C. Randomized study on the efficacy of immunosuppressive therapy in patients with virus-negative inflammatory cardiomyopathy: the TIMIC study. Eur Heart J 2009;30: 1995-2002.
553. Robinson J, Hartling L, Vandermeer B, Klassen TP. Intravenous immunoglobulin for presumed viral myocarditis in children and adults. Cochrane Database Syst Rev 2015:CD004370.
554. Kishimoto C, Shioji K, Hashimoto T, et al. Therapy with immunoglobulin in patients with acute myocarditis and cardiomyopathy: analysis of leukocyte balance. Heart Vessels 2014;29:336-42.
555. Goland S, Czer LS, Siegel RJ, et al. Intravenous immunoglobulin treatment for acute fulminant inflammatory cardiomyopathy: series of six patients and review of literature. Can J Cardiol 2008;24:571-4.
556. Wagner FM, Hraska V, Doering V, et al. Bridge to recovery by mechanical ventricular assist (VAD) e a successful therapy for cardiac failure due to acute myocarditis (AM). J Heart Lung Transplant 2006;25:S123.
557. Acker MA. Mechanical circulatory support for patients with acutefulminant myocarditis. Ann Thorac Surg 2001;71:S73-6 [discussion: S82-S85].
558. Wagner A, Schulz-Menger J, Dietz R, Friedrich MG. Long-term followup of patients paragraph sign with acute myocarditis by magnetic paragraph sign resonance imaging. MAGMA 2003;16:17-20.
