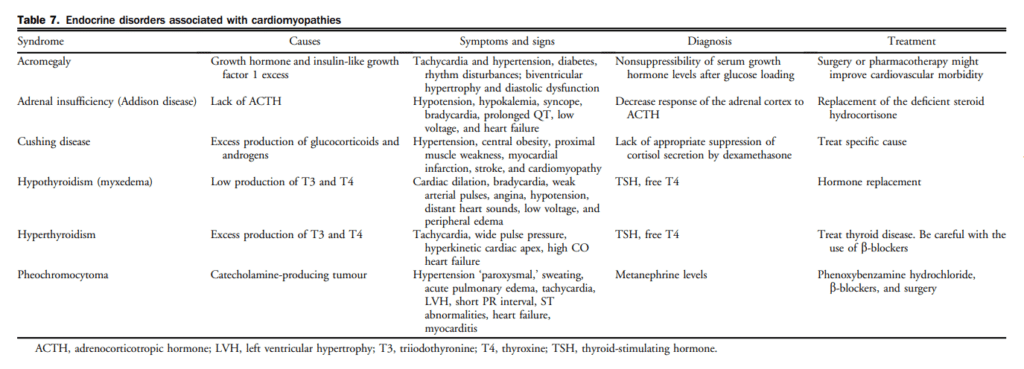
6. Biomarkers/NPs
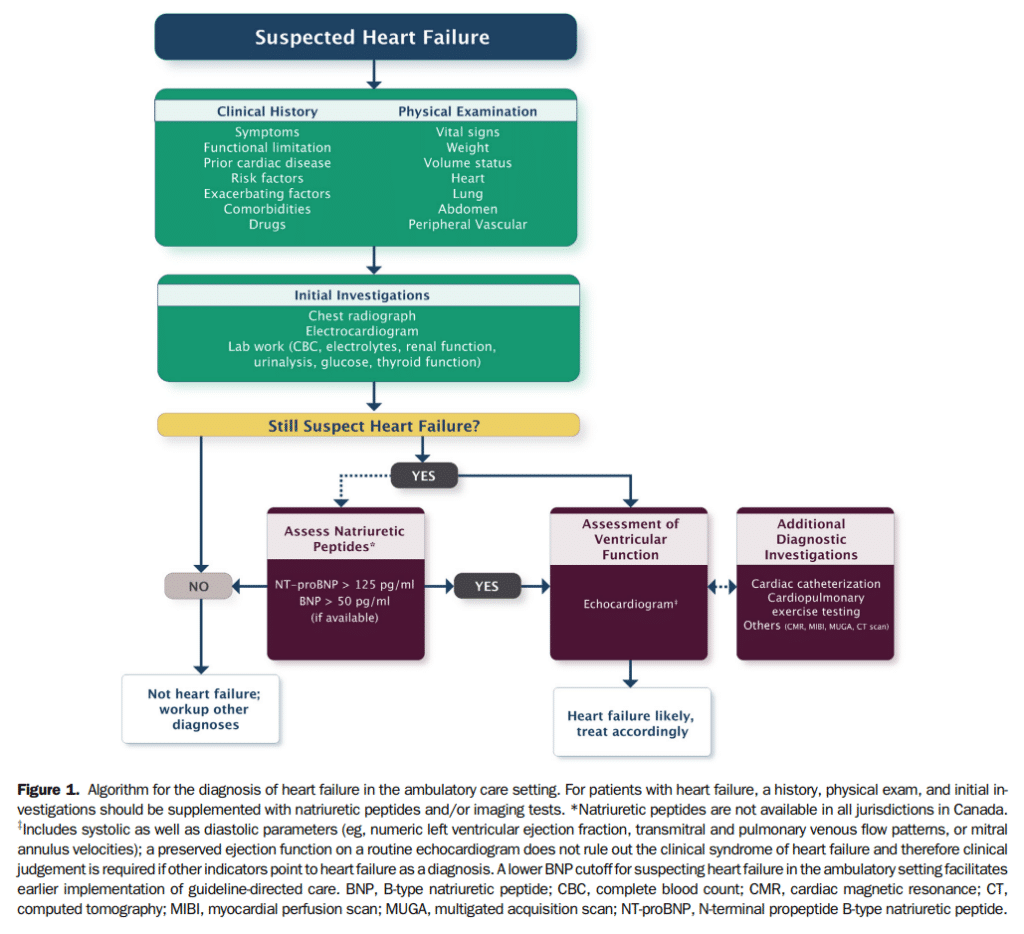
Biomarkers, for the context of this guideline, refer to substances measured in the blood other than commonly used laboratory tests and imaging studies. Several general criteria have been proposed for what constitutes a relevant biomarker in cardiovascular medicine.[58] Over the past decade, the NPs became the gold standard for biomarkers in HF and have been extensively investigated in various clinical settings. NPs might be elevated in relation with other cardiovascular conditions leading to increased LV filling pressures, such as valvular heart disease, ischemia, or uncontrolled hypertension.[59] Noncardiac conditions, such as increasing age, renal dysfunction, anemia, pulmonary diseases, and sepsis have also been associated with increased NP levels.[59],[60] Obesity has been associated with lower NP levels.[61] The prognostic utility of NPs has been shown in HF.[62]–[65] The availability of NPs in Canada remains challenging because of the associated costs and/or the perceived variable effect on clinical decisions.[66] The use of NPs does not eliminate the need for cardiac imaging in most cases. Hence, NPs provide additional evidence in favour of HF but need to be placed within the clinical context (Fig. 1).
6.1 NPs and optimization of medical therapy
One of the reasons for the so-called “mismatch” between risk and treatment is the lack of reliable markers to guide the titration of effective treatments. Persistently elevated or increasing NP levels are associated with an increased risk of hospitalization and mortality. In otherwise clinically stable patients with HF, a change in NP levels ≥ 30% between visits indicates a change greater than would be expected from daily variation[67] and is likely clinically relevant and should therefore call for more intensive follow-up and/or intensified medical treatments.
Data suggest that serial monitoring of NP levels can provide powerful information about response to therapy and residual risk.[68]–[70] Initial studies on NP-guided therapy[71]–[73] have targeted a large reduction or a very low NP level in the intervention group; and generally compared this intervention with contemporary guideline-directed medical therapy (GDMT). Targeting a specific reduction in NP levels (or “NP-guided therapy”) has shown an improvement in clinical outcomes, although these studies were smaller and ongoing studies will provide further guidance.[74],[75]
NP concentrations have been shown to decrease in response to commonly used therapies for either acute or chronic HF. This includes loop diuretics, ACE inhibitors (ACEis), ARBs, mineralocorticoid receptor antagonists (MRAs), and CRT.[73],[76],[77] With β-blockers, an initial increase in NP levels might be seen during the first 8-12 weeks followed by a decrease.[78] The interaction between NP levels and neprilysin inhibitors is more complex but evidence suggests that NT-proBNP might more reliably reflect the patient status at least in the first 8 months of treatment with sacubitril/valsartan (Fig. 3).[79]
6.1.1 HFpEF and NPs
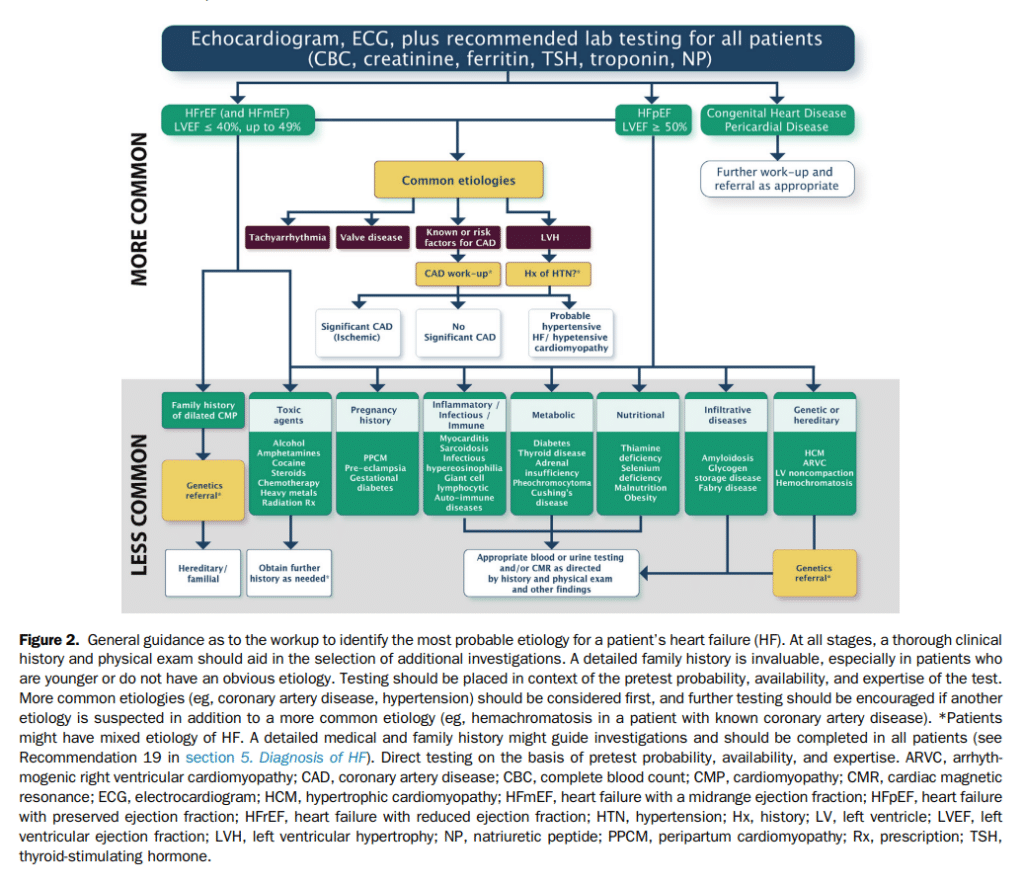
Although elevated NP levels have been proposed as an additional diagnostic criterion for HFpEF,[80] older age and comorbidities common in this population might also influence NP levels.[81] Among patients with HFpEF, the presence of an elevated NP level is an established marker of risk and discriminates prognosis comparable with that of HFrEF.[82] In the subset of 375 patients randomized in the Perindopril in Elderly People with Chronic Heart Failure Trial (PEP-CHF) with available measurements of NT-proBNP at baseline, those in the highest quartile (> 1035 pg/mL) had more than a fourfold risk of all-cause mortality or HF-related hospitalization over those in the lowest quartile (< 176 pg/mL), and this relationship was independent of therapy.[83] Similarly, in the larger cohort of 3480 patients with measured NT-proBNP levels in the Irbesartan in Patients With Heart Failure and Preserved Ejection fraction (I-PRESERVE) trial, values above the median of 339 pg/mL at baseline were independently associated with a twofold increase in risk of all-cause mortality.[84] In the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial, patients randomized in the trial on the basis of elevated NPs derived outcome benefits from spironolactone, whereas those randomized on the basis of a previous hospitalization for HF did not.[31] These findings might have been influenced by significant regional variations in the trial[85] but nevertheless, they show the utility of NPs in selecting patients who might respond to a specific treatment.[86]
6.1.2 NPs for the diagnosis and management of HF
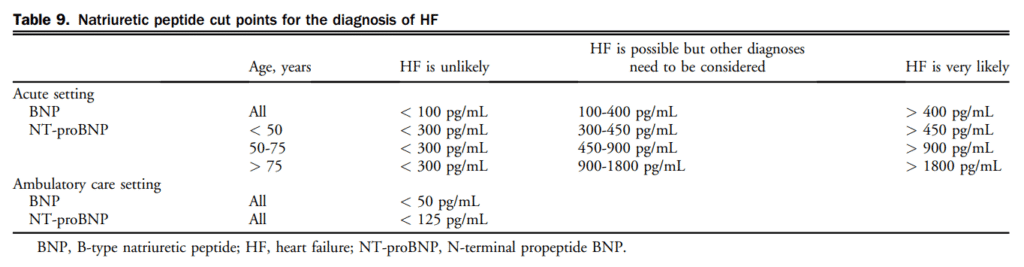
The levels of NPs for ruling in and ruling out a diagnosis of HF are shown in Table 9. NP levels differ for the diagnosis of patients seen in the acute (eg, emergency department [ED]) vs in the outpatient settings. Several high-quality studies have reported on the utility of NPs for the diagnosis of HF in the outpatient setting where NPs are ideally suited to assist in ruling out HF as a diagnosis, but cannot be used independent of signs, symptoms, and other diagnostic information.
Recommendation
21. We recommend that BNP/NT-proBNP levels be measured to help confirm or rule out a diagnosis of HF in the acute or ambulatory care setting in patients in whom the cause of dyspnea is in doubt (Strong Recommendation; High-Quality Evidence).
Values and Preferences
High-quality RCT evidence in the Canadian setting also shows favourable cost-effectiveness. Elevated NP levels are recommended as an additional diagnostic criterion for HFpEF and are associated with increased risk, although the levels might be lower than in HFrEF. Older age and comorbidities might also influence variations in NP levels.
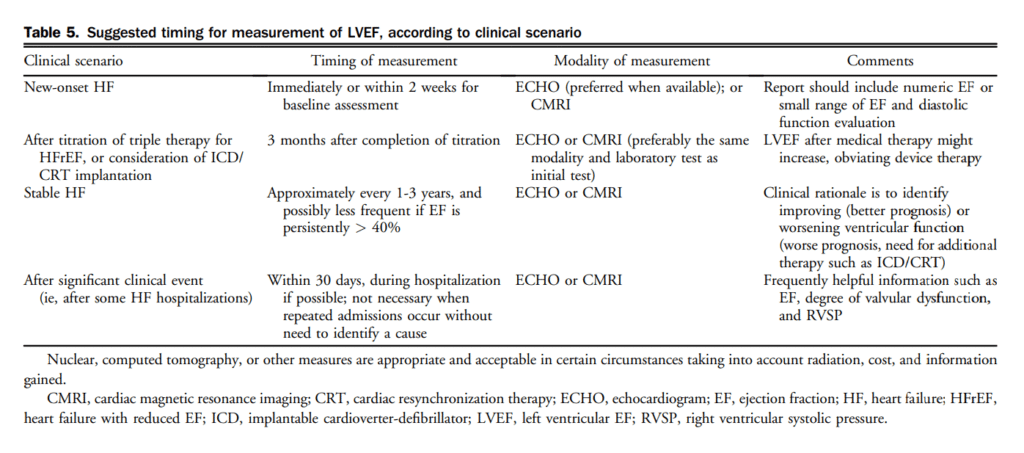
22. We recommend that measurement of BNP/NT-proBNP levels be considered in patients with an established diagnosis of HFrEF for prognostic stratification, in view of optimizing medical therapy (Strong Recommendation; High-Quality Evidence).
Practical Tip
For patients receiving an angiotensin receptor-neprilysin inhibitor (ARNI) (see section 7.1.1.5. ARNI), the use of NT-proBNP (rather than BNP) should be preferred to evaluate prognosis during the first year of treatment. BNP levels will be increased as a consequence of the ARNI’s mechanisms of action over at least the first 8 months of treatment.
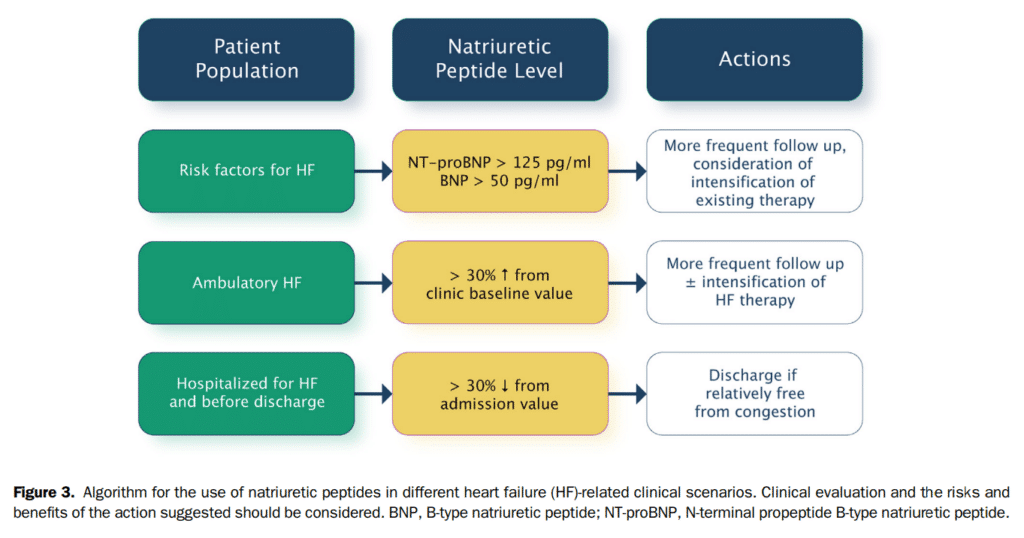
6.1.3 NPs for the management of chronic HFrEF
Recommendation
management should be considered to decrease HF-related hospitalizations and potentially reduce mortality. The benefit is uncertain in individuals older than 75 years of age (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendations are on the basis of multiple small RCTs, most of which showed benefit, and 3 meta-analyses, which universally showed benefit. An ongoing RCT is likely to affect this recommendation.
Practical Tip
A change in NP levels by > 30% probably reflects more than daily variation in patients with compensated HF.
Practical Tip
The timing of NP measurements in outpatient settings should be dictated according to clinical status; NP measurements should be used when they might aid in clinical decision-making.
6.1.4 NPs for the management of decompensated chronic HFrEF
24. We suggest that measurement of BNP or NT-proBNP in patients hospitalized for HF should be considered before discharge, because of the prognostic value of these biomarkers in predicting rehospitalization and mortality (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation is on the basis of multiple small RCTs, all of which showed an association with clinical outcomes.
Practical Tip
A patient with persistently elevated NP levels might need closer follow-up to reduce the risk of rehospitalization.
Practical Tip
For patients who are about to be discharged from the hospital after a HF hospitalization, the NP level should be lower than that on admission. If NP levels remain elevated, clinicians should re-evaluate the patient’s condition and consider the possibility of delaying discharge from the hospital to optimize therapy and further reduce the NP level.
6.1.5 Myocardial injury, myocyte death, and troponins
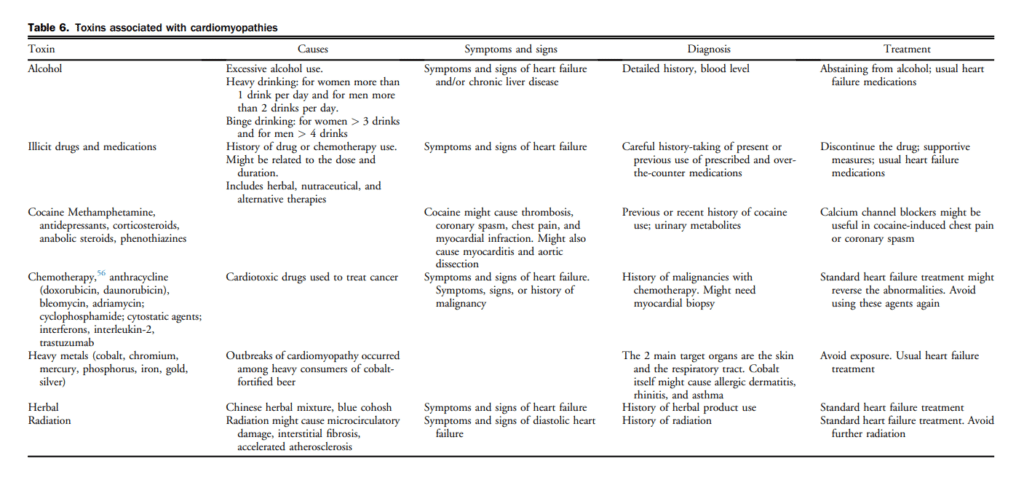
In the Valsartan in Heart Failure Trial (Val-HeFT), 10.4% of subjects had detectable cardiac troponin T (cTnT) with a fourth-generation clinical assay (detection limit 0.01 ng/mL) and this proportion increased to 92% when an hs assay (hs-cTnT; detection limit 0.001 ng/mL) was used.[87] Although the pathophysiology of cardiac troponin release in HF remains uncertain, several factors including subendocardial ischemia and myocyte necrosis, cardiomyocyte damage from inflammatory cytokines, or oxidative stress, apoptosis, and leakage of troponin from the cytosolic pool due to increased membrane permeability have been invoked (Table 10).[88] The degree of troponin elevation is a powerful predictor of mortality and cardiovascular events in ambulatory as well as acutely decompensated patients with chronic HFrEF, even after adjustment for traditional risk predictors including NPs.[87],[89],[90] Limited data are available regarding the prognostic significance of cTnT elevations in the ambulatory population with HFpEF, although levels do appear to be elevated to an extent comparable with that seen in HFrEF.[91] In an analysis of the Acute Decompensated Heart Failure National Registry (ADHERE) of 84,872 patients hospitalized with acutely decompensated congestive HF, patients with positive cardiac troponins had a higher in-hospital mortality independent of other predictive variables in patients with HF.[92] Latini et al tested the prognostic value of the hs-cTnT assay in 4053 patients with chronic HF and showed that cTnT was detectable in 10.4% with the currently available assay compared with 92% using the hs-cTnT assay. Patients with hs-cTnT levels above the median had more severe HF and worse outcomes.[87]
Recommendation
commend that high-sensitivity (hs) troponins be measured on admission for AHF, to rule out ACS and for prognostic stratification (Strong Recommendation; High-Quality Evidence).
Values and Preferences
The degree of hs troponin elevation is a powerful predictor of mortality and cardiovascular events in ambulatory as well as acutely decompensated patients with chronic HFrEF, even after adjustment for traditional risk predictors including NPs. However, it is yet unclear how the use of serial hs troponin measurements in addition to NPs for HFrEF management would provide additional and cost-effective benefits in terms of improving outcomes. Also, limited data are available regarding the prognostic significance of hs troponin elevations in ambulatory patients with HFpEF.
References
58. Ahmad T, Fiuzat M, Pencina MJ, et al. Charting a roadmap for heart failure biomarker studies. JACC Heart Fail 2014;2:477-88.
59. Burke MA, Cotts WG. Interpretation of B-type natriuretic peptide in cardiac disease and other comorbid conditions. Heart Fail Rev 2007;12: 23-36.
60. Thygesen K, Mair J, Mueller C, et al. Recommendations for the use of natriuretic peptides in acute cardiac care: a position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur Heart J 2012;33:2001-6.
61. Daniels LB, Clopton P, Bhalla V, et al. How obesity affects the cutpoints for B-type natriuretic peptide in the diagnosis of acute heart failure. Results from the Breathing Not Properly Multinational Study. Am Heart J 2006;151:999-1005.
62. Moe GW, Ezekowitz JA, O’Meara E, et al. The 2014 Canadian Cardiovascular Society heart failure management guidelines focus update: anemia, biomarkers, and recent therapeutic trial implications. Can J Cardiol 2015;31:3-16.
63. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail 2016;18:891-975.
64. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128:e240-327.
65. van Veldhuisen DJ, Linssen GC, Jaarsma T, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 2013;61:1498-506.
66. Sepehrvand N, Bakal JA, Lin M, et al. Factors associated with natriuretic peptide testing in patients presenting to emergency departments with suspected heart failure. Can J Cardiol 2016;32:986.e1-8.
67. Troughton R, Michael Felker G, Januzzi JL Jr. Natriuretic peptideguided heart failure management. Eur Heart J 2014;35:16-24.
68. Masson S, Latini R, Anand IS, et al. Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the Valsartan Heart Failure (Val-HeFT) data. Clin Chem 2006;52: 1528-38.
69. Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ 2005;330:625.
70. Cleland JG, McMurray JJ, Kjekshus J, et al. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure). J Am Coll Cardiol 2009;54: 1850-9.
71. Berger R, Moertl D, Peter S, et al. N-terminal pro-B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol 2010;55:645-53.
72. Sanders-van Wijk S, Muzzarelli S, Neuhaus M, et al. Safety and tolerability of intensified, N-terminal pro brain natriuretic peptide-guided compared with standard medical therapy in elderly patients with congestive heart failure: results from TIME-CHF. Eur J Heart Fail 2013;15:910-8.
73. Motiwala SR, Januzzi JL Jr. The role of natriuretic peptides as biomarkers for guiding the management of chronic heart failure. Clin Pharmacol Ther 2013;93:57-67.
74. Stienen S, Salah K, Moons AH, et al. Rationale and design of PRIMA II: a multicenter, randomized clinical trial to study the impact of inhospital guidance for acute decompensated heart failure treatment by a predefined NT-PRoBNP target on the reduction of readmIssion and Mortality rAtes. Am Heart J 2014;168:30-6.
75. Felker GM, Ahmad T, Anstrom KJ, et al. Rationale and design of the GUIDE-IT study: guiding evidence based therapy using biomarker intensified treatment in heart failure. JACC Heart Fail 2014;2:457-65.
76. Cleland J, Freemantle N, Ghio S, et al. Predicting the long-term effects of cardiac resynchronization therapy on mortality from baseline variables and the early response a report from the CARE-HF (Cardiac Resynchronization in Heart Failure) Trial. J Am Coll Cardiol 2008;52: 438-45.
77. Shanmugam N, Campos AG, Prada-Delgado O, et al. Effect of atrioventricular optimization on circulating N-terminal pro brain natriuretic peptide following cardiac resynchronization therapy. Eur J Heart Fail 2013;15:534-42.
78. Davis ME, Richards AM, Nicholls MG, et al. Introduction of metoprolol increases plasma B-type cardiac natriuretic peptides in mild, stable heart failure. Circulation 2006;113:977-85.
79. Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015;131: 54-61.
80. Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539-50.
81. Jeevanantham V, Shrivastava R, Nannapaneni S, et al. Elevated B-type natriuretic peptide level: use with caution in patients with multiple comorbidities and presenting with dyspnea. Indian Heart J 2007;59:64-8.
82. Carlsen CM, Bay M, Kirk V, et al. Prevalence and prognosis of heart failure with preserved ejection fraction and elevated N-terminal pro brain natriuretic peptide: a 10-year analysis from the Copenhagen Hospital Heart Failure Study. Eur J Heart Fail 2012;14:240-7.
83. Cleland JG, Taylor J, Freemantle N, et al. Relationship between plasma concentrations of N-terminal pro brain natriuretic peptide and the characteristics and outcome of patients with a clinical diagnosis of diastolic heart failure: a report from the PEP-CHF study. Eur J Heart Fail 2012;14:487-94.
84. Anand IS, Rector TS, Cleland JG, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail 2011;4:569-77.
85. Pfeffer MA, Claggett B, Assmann SF, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015;131:34-42.
86. Ibrahim NE, Gaggin HK, Konstam MA, Januzzi JL Jr. Established and emerging roles of biomarkers in heart failure clinical trials. Circ Heart Fail 2016;9:e002528.
87. Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007;116:1242-9.
88. Kociol RD, Pang PS, Gheorghiade M, et al. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol 2010;56:1071-8.
89. Pascual-Figal DA, Manzano-Fernandez S, Boronat M, et al. Soluble ST2, high-sensitivity troponin T- and N-terminal pro-B-type natriuretic peptide: complementary role for risk stratification in acutely decompensated heart failure. Eur J Heart Fail 2011;13:718-25.
90. Masson S, Anand I, Favero C, et al. Serial measurement of cardiac troponin T using a highly sensitive assay in patients with chronic heart failure: data from 2 large randomized clinical trials. Circulation 2012;125:280-8.
91. Santhanakrishnan R, Chong JP, Ng TP, et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur J Heart Fail 2012;14:1338-47.
92. Peacock WF, De Marco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med 2008;358:2117-26.
