4. Prevention of HF and Asymp
4.1 Early detection of LVSD and prevention of HF
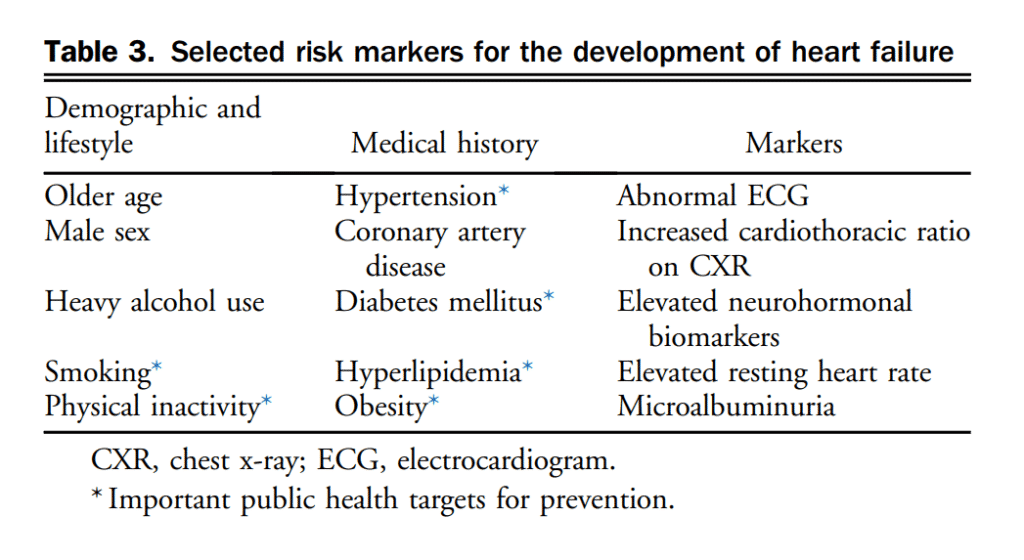
HF often progresses from asymptomatic LVSD to symptomatic HF.[16] Early detection of LVSD might allow intervention on contributing risk factors and pharmacotherapy to delay or reverse the progression of adverse LV remodelling. Data on medications, including ACEs, ARBs, and β-blockers are summarized online in evidence reviews at www.ccs.ca. Conventional risk factors for cardiovascular disease (CVD) are often included in clinical assessment but a detailed family history might also uncover genetic causes or susceptibility to the development of LV dysfunction. The use of NPs might be useful to identify individuals who are at higher risk for the development of HF and in whom preventative strategies have been studied. The cut point used in the Saint Vincent Screening to Prevent Heart Failure (STOP-HF)[17] trial of BNP > 50 pg/mL to undergo echocardiography and collaborative care resulted in a higher rate of use of renin-angiotensin-aldosterone system inhibition therapies, fewer HF events, and significant reduction in hospitalizations for major cardiovascular events over a follow-up on an average of 4.2 years. The NT-proBNP Selected Prevention of Cardiac Events in a Population of Diabetic Patients Without a History of Cardiac Disease (PONTIAC) study[18] used a cut point of NT-proBNP > 125 pg/mL to apply further cardiology consultation and individualized β-blockade and renin-angiotensin-aldosterone system uptitration. Patients in the group randomized to intensified therapy had a 65% relative risk reduction (RRR) in the primary combined event rate of hospitalization or death due to cardiac disease at 2 years. Therapies used in these 2 trials are guideline-based, reinforcing the opportunity to enhance neurohormonal therapy in all individuals with cardiovascular risk factors, limited only by the availability of NP measurement to identify patients. Exercise as a strategy to prevent ischemic heart disease has supported guideline recommended minimum physical activity of at least 150 minutes per week of moderate intensity activity (approximately 500 metabolic equivalents of task minutes). A meta-analysis of 12 prospective cohort studies by Pandey et al.[19] reported the risk of HF is reduced by 10%, 19%, and 35% in people who were participating in leisure activity of 500, 1000, and 2000 metabolic equivalents of task minutes per week, respectively, compared with individuals with no physical activity. This article noted an inverse dose-response relationship between physical activity and development of HF. The importance of prevention of HF is supported by evidence that preventing and treating cardiovascular risk factors and conditions that cause atherosclerotic disease leads to fewer patients developing HF. Many of these risk factors also contribute to the development of HF independently from atherosclerotic disease (Table 3). Previous HF guidelines have reviewed the substantial evidence supporting the screening and management of common risk factors for the development of HF such as hypertension, diabetes, smoking, dyslipidemia, obesity, alcohol use, and sedentary behaviour.[20]–[27] Patients with established coronary artery disease (CAD) and/or previous acute coronary syndrome (ACS) should have these appropriately treated to prevent future HF events.
Recommendation
1. We suggest clinical assessment in all patients to identify known or potential risk factors for the development of HF (Weak Recommendation; Moderate-Quality Evidence).
2. We recommend an angiotensin-converting enzyme (ACE) inhibitor (ACEi) be used in all asymptomatic patients with an EF < 35% (Strong Recommendation; Moderate-Quality Evidence).
3. We recommend that an ACEi should be prescribed in established effective doses to reduce the risk of developing HF in patients with evidence of vascular disease or diabetes with end organ damage (Strong Recommendation; High-Quality Evidence).
4. We recommend that in ACE-intolerant patients, an angiotensin receptor blocker (ARB) be considered for reduction of the risk of developing HF in patients with evidence of vascular disease or diabetes with end organ damage (Strong Recommendation; High-Quality Evidence).
5. We recommend that health professionals caring for overweight or obese individuals should educate them about the increased risk of HF (Strong Recommendation; Moderate-Quality Evidence).
6. We recommend physical activity to reduce the risk of developing HF in all individuals (Strong Recommendation; Moderate-Quality Evidence).
Practical Tip
Natriuretic peptide (NP) screening of individuals at risk for the development of HF can aid decision making on whom to send for echocardiography. A value of B-type (BNP) > 50 pg/mL or N-terminal propeptide BNP (NT-proBNP) > 125 pg/mL should prompt a request for specialist consultation and imaging, and/or initiation or intensification of neurohormonal blocking agents and lifestyle interventions.
Practical Tip
Dyslipidemia should be treated in patients with evidence of vascular disease or diabetes with lipid-lowering drugs, especially statins.
Practical Tip
Smoking cessation, improved cardiorespiratory fitness, and weight reduction for overweight or obese individuals are important preventive strategies for HF.
Practical Tip
Patients at high risk for developing HF should receive annual influenza vaccine and periodic pneumococcal pneumonia immunizations.
4.2 Preventing HF in patients with hypertension
Hypertension has been well documented as a risk factor for HF and the treatment of hypertension has been shown to reduce the risk of developing HF.[28]–[30] In addition to the high-quality meta-analyses, more recent evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) supports a more aggressive approach to hypertension management.[31] This trial of 9361 participants deemed high risk for a cardiovascular event, randomized to intensive (systolic BP < 120 mm Hg) vs standard (systolic BP < 140 mm Hg) BP control showed there was a 25% risk reduction in the primary outcome of myocardial infarction (MI), ACS, stroke, HF, or death from cardiovascular causes after only a median of 3.26 years. There was a 33% reduction of future HF outcomes (patients with a history of symptomatic HF in the past 6 months or with LVEF < 35% were excluded). Readers are directed to Hypertension Canada’s 2017 Guidelines (http://www.onlinecjc.ca/article/S0828-282X(17)30110-1/fulltext) for additional information.
Recommendation
7. We recommend that most patients should have their blood pressure (BP) controlled to < 140/90 mm Hg; those with diabetes or at high risk for cardiovascular events should be treated to a systolic BP of < 130 mm Hg to reduce the risk of developing HF (Strong Recommendation; Moderate-Quality Evidence).
8. We recommend that β-blockers should be considered in all asymptomatic patients with an LVEF < 40% (Strong Recommendation; Moderate-Quality Evidence).
4.3 Preventing HF in patients with diabetes
Diabetes mellitus (DM) is an established risk factor for the development of HF.[29],[32],[33] However, the relationship between glycemic control and the development of HF is inconsistent and complicated further by the long-term effects of diabetes on other organ systems (eg, kidneys) or development of CAD.[34] It is recognized, however, that DM can produce HF independently of CAD by causing a diabetic cardiomyopathy.[29] In several studies, the incidence of HF was two- and fourfold higher in patients with DM than in those without.[32],[33],[35]–[38] Approximately 12% of DM patients have HF,[35] and older than the age of 64 years, the prevalence increases to 22%.[37] It is thought that diabetes promotes the development of myocardial fibrosis and diastolic dysfunction, autonomic dysfunction, and worsened renal and endothelial function. Moreover, there has been uncertainty regarding whether any glucose-lowering strategy, or specific therapeutic agent, is safe from a cardiovascular standpoint or can decrease cardiovascular risk. Older trials on the effects on cardiovascular outcomes of specific glucose-lowering strategies or medications either have been insufficiently powered or have shown no significant cardiovascular benefit or an increased risk of death or HF.
4.3.1 Glycemic control in diabetes to prevent HF
In the past, several diabetes guidelines have advocated for tight glycemic control (lower Hb A1c); however, there is no evidence that this approach improves cardiovascular outcomes and some studies suggest harm, including increased HF, not to mention increased risk for hypoglycemia. There are no specific studies targeting patients with HF. Data are largely extrapolated from the Diabetes Control and Complications Trial (DCCT) study of patients with type 1 diabetes,[39] the UK Prospective Diabetes Study (UKPDS) study,[40] the UKPDS Follow Up study,[41] the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study,[42],[43] the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR-Controlled Evaluation (ADVANCE) study,[44] and the Veterans Affairs Diabetes Trial (VADT) study.[45] With the available evidence, an intensive glycemic control strategy cannot be recommended for all patients with diabetes. Instead, each individual should be assessed for his or her optimal glycemic target for the prevention of macrovascular events or HF.
Recommendation
9. We recommend that diabetes should be treated according to the Canadian Diabetes Association’s national guidelines to achieve optimal control of blood glucose levels (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
There is no convincing evidence from randomized controlled trials (RCTs) that tighter glycemic control reduces cardiovascular outcomes. Potential risks of tight glycemic control might outweigh its benefits in certain individuals such as those with a long duration of diabetes, frequent episodes of hypoglycemia, those with advanced CVD, advanced age, frailty, or multiple comorbidities.
Practical Tip
Each individual patient should be assessed for his or her “optimal” glycemic control hemoglobin (Hb) A1c target. Considerations include an individual’s risk of hypoglycemia, the duration of diabetes, the presence or absence of CVD, kidney function, overweight or not, or frailty, among others.
Metformin. Metformin is still considered first-line pharmacological therapy for type 2 diabetes. It is effective, has a known safety profile, and is well tolerated in patients with HF.[46]
Recommendation
10. We suggest that metformin might be considered a first-line agent for type 2 diabetes treatment (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
Metformin is the current Canadian Diabetes Association first-line treatment for type 2 diabetes.
Practical Tip
If the estimated glomerular filtration rate (eGFR) is < 30 mL/min, a temporary discontinuation of metformin and certain other diabetes medications should be considered.
SGLT-2 inhibitors. The Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients-Removing Excess Glucose (EMPA-REG OUTCOME) trial,[47] is an RCT to show cardiovascular benefit in the treatment of diabetes. A sodium-glucose co-transporter-2 (SGLT-2) inhibitor empagliflozin was compared with placebo in 7020 patients with type 2 diabetes and established CVD and eGFR ≥ 30 mL/min. The primary composite outcome was death from cardiovascular causes, nonfatal MI, or nonfatal stroke. The primary end point occurred less commonly in the patients treated with empagliflozin (10.5%) than in those who received placebo (hazard ratio [HR], 12.1%; 95% confidence interval [CI], 0.74-0.99). Moreover, empagliflozin had an RRR for cardiovascular mortality of 38%, all-cause mortality of 32%, and HF hospitalization of 35%. SGLT-2 inhibitors have not yet been studied in populations of patients with HF. Of note, only a subgroup of approximately 10% of patients in the EMPA-REG OUTCOME trial had a reported history of HF. There are ongoing trials of SGLT-2 inhibitors, and those might affect future recommendations, namely for patients with established HF.
Recomendation
11. We suggest that the use of empagliflozin, an SGLT-2 inhibitor, be considered for patients with type 2 diabetes and established CVD for the prevention of HF-related outcomes (Weak Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation places weight on the fact that empagliflozin is the first diabetes-related medication to show a reduction in HF hospitalization. Empagliflozin was well tolerated and associated with an acceptable side effect profile within the clinical trial establishing its efficacy and safety. There are ongoing trials of this class of medications that might change this recommendation.
DPP-4 inhibitors. The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellituse Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53) trial[48] randomized 16,492 patients with type 2 diabetes who had a history of, or were at risk for, cardiovascular events to receive the dipeptidyl peptidase-4 (DPP-4) inhibitor saxagliptin or placebo and followed them for a median of 2.1 years. The primary end point was a composite of cardiovascular death, MI, or ischemic stroke. At a median follow-up of 2.1 years, rates of composite cardiovascular events were similar with saxagliptin and placebo, but hospitalization for HF was higher with saxagliptin (3.5% vs 2.8%; HR, 1.27; P = 0.007). In contrast, the Trial Evaluating Cardiovascular Outcomes With Sitagliptin (TECOS) study,[49] an RCT of 14,671 patients comparing another DPP-4 inhibitor, sitagliptin, with placebo showed no increase in HF hospitalization (HR, 1.00; 95% CI, 0.83-1.20; P = 0.98). Studies involving other DPP-4 inhibitors alogliptin[50] and linagliptin[51] have not shown additional increases in the risk of HF events.
Recommendation
12. We do not recommend the use of the DPP-4 inhibitor saxagliptin in patients with or at risk for HF (Strong Recommendation; Moderate-Quality Evidence).
13. We suggest that if a DPP-4 inhibitor is to be used, linagliptin or sitagliptin should be considered for patients with diabetes and with, or at risk for HF (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
The Saxagliptin Assessment of Vascular Outcomes Recorded in Patients With Diabetes Mellitus (SAVOR) trial showed an increase in HF hospitalizations with use of saxagliptin. Other DPP-4 inhibitors (eg, sitagliptin, alogliptin, linagliptin) did not have the same adverse effect as saxagliptin of HF hospitalization; there are ongoing trials of other DPP-4 inhibitors.
Glucagon-like peptide. Human glucagon-like peptide (GLP-1) agonists have been tested in patients with diabetes for the outcomes of cardiovascular events. One such agent, liraglutide, was tested in the Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial, and 14% of the patients had a clinical history of HF. Overall, liraglutide was shown to be noninferior to placebo, and had fewer cardiovascular events overall. There was no statistically significant decrease or increase in the number of HF events. There are ongoing trials with other GLP-1 agonists that will inform a recommendation on this class of agents for the prevention of HF.[52]
Thiazolidinediones. Two such drugs (pioglitazone and rosiglitazone) have each been shown to increase the risk of HF events.
Prospective Pioglitazone Clinical Trial in Macro Vascular Events (PROactive)[53] was a randomized study of 5238 type 2 diabetic patients, comparing pioglitazone with placebo. More pioglitazone (5.7%) than placebo patients (4.1%) had a serious HF event during the study (P = 0.007). Of patients in the placebo group, 108 needed hospital admission for HF (153 admissions) compared with 149 (209 admissions) in the pioglitazone group (HR, 1.41; 95% CI, 1.10-1.80; P = 0.007).
Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD)[54] was an RCT of 4447 people with type 2 diabetes randomized to add-on rosiglitazone (n = 2220) or to a combination of metformin and sulfonylurea (n = 2227). Patients with any HF were excluded. Rosiglitazone-treated patients had a greater risk for at least 1 admission to hospital for HF compared with the placebo group (HR, 2.6; 95% CI, 1.1-4.1; P = 0.001). A meta-analysis of 42 trials of rosiglitazone[55] showed a 43% increase in MI and a 64% increase in death from cardiovascular causes with use of rosiglitazone.
Recommendation
14. We recommend that thiazolidinediones should not be used in patients with HF (Strong Recommendation; High-Quality Evidence).
References
16. Wang TJ, Evans JC, Benjamin EJ, et al. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation 2003;108:977-82.
17. Ledwidge M, Gallagher J, Conlon C, et al. Natriuretic peptide-based screening and collaborative care for heart failure: the STOP-HF randomized trial. JAMA 2013;310:66-74.
18. Huelsmann M, Neuhold S, Resl M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol 2013;62:1365-72.
19. Pandey A, Garg S, Khunger M, et al. Dose-response relationship between physical activity and risk of heart failure: a meta-analysis. Circulation 2015;132:1786-94.
20. Eriksson H, Svardsudd K, Larsson B, et al. Risk factors for heart failure in the general population: the study of men born in 1913. Eur Heart J 1989;10:647-56.
21. Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 1993;22:6A-13A.
22. Nicklas BJ, Cesari M, Penninx BW, et al. Abdominal obesity is an independent risk factor for chronic heart failure in older people. J Am Geriatr Soc 2006;54:413-20.
23. Kenchaiah S, Gaziano JM, Vasan RS. Impact of obesity on the risk of heart failure and survival after the onset of heart failure. Med Clin North Am 2004;88:1273-94.
24. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305-13.
25. Aune D, Sen A, Norat T, et al. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and doseresponse meta-analysis of prospective studies. Circulation 2016;133: 639-49.
26. Baena-Diez JM, Byram AO, Grau M, et al. Obesity is an independent risk factor for heart failure: Zona Franca Cohort study. Clin Cardiol 2010;33:760-4.
27. Fitchett D, Zinman B, Wanner C, et al. Heart failure outcomes with empagliflozin in patients with type 2 diabetes at high cardiovascular risk: results of the EMPA-REG OUTCOME(R) trial. Eur Heart J 2016;37: 1526-34.
28. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA 1996;275: 1557-62.
29. Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 2003;26:2433-41.
30. Turnbull F. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003;362:1527-35.
31. Williamson JD, Supiano MA, Applegate WB, et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged 75 years: a randomized clinical trial. JAMA 2016;315: 2673-82.
32. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974;34:29-34.
33. He J, Ogden LG, Bazzano LA, et al. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med 2001;161:996-1002.
34. Sharma A, Ezekowitz JA. Diabetes, impaired fasting glucose, and heart failure: it’s not all about the sugar. Eur J Heart Fail 2014;16:1153-6.
35. Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 2004;27:1879-84.
36. Thrainsdottir IS, Aspelund T, Thorgeirsson G, et al. The association between glucose abnormalities and heart failure in the population-based Reykjavik study. Diabetes Care 2005;28:612-6.
37. Bertoni AG, Hundley WG, Massing MW, et al. Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care 2004;27:699-703.
38. Johansson S, Wallander MA, Ruigomez A, Garcia Rodriguez LA. Incidence of newly diagnosed heart failure in UK general practice. Eur J Heart Fail 2001;3:225-31.
39. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993;329:977-86.
40. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998;352:837-53.
41. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89.
42. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59.
43. Group AS, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818-28.
44. Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-72.
45. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360: 129-39.
46. MacDonald MR, Eurich DT, Majumdar SR, et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested casecontrol study from the U.K. General Practice Research Database. Diabetes Care 2010;33:1213-8.
47. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373: 2117-28.
48. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26.
49. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373: 232-42.
50. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385:2067-76.
51. Rosenstock J, Marx N, Neubacher D, et al. Cardiovascular safety of linagliptin in type 2 diabetes: a comprehensive patient-level pooled analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol 2015;14:57.
52. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375: 311-22.
53. Erdmann E, Charbonnel B, Wilcox RG, et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08). Diabetes Care 2007;30:2773-8.
54. Komajda M, McMurray JJ, Beck-Nielsen H, et al. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J 2010;31:824-31.
55. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457-71.
