5. Screening and Opportunistic AF Detection
5.1 Opportunistic AF detection in the general population
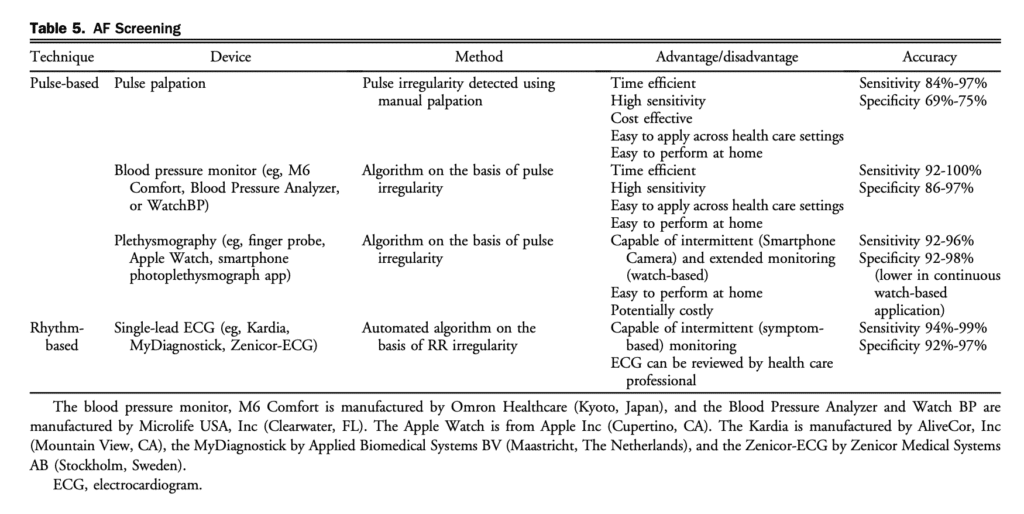
AF screening initiatives have emerged with the availability of safe and effective stroke prevention therapy, well defined stroke risk schemes, and new technologies that have simplified AF monitoring. Because a large number of patients with AF might be asymptomatic, screening might provide an opportunity for AF detection with early initiation of stroke prevention therapy to reduce the risk of AF-related complications. The effects of screening (eg, opportunistic case finding or systematic screening) has been examined in numerous studies in various populations (eg, general population or high-risk subgroups) and settings (eg, community, outpatient clinics, or inpatient).[81],[82] Taken together, the rate of new AF detection was 0.9% (95% confidence interval [CI], 0.7-1.1) across 23 prospective cross sectional studies, yielding a number needed to screen (NNS) of 111 individuals to detect 1 patient with AF.[81] However, the prevalence of AF varies as a function of age and sex. When age and sex adjustment was performed the AF detection rate after a single time point screen was noted to be 1.44% (95% CI, 1.13-1.82) for those 65 years of age or older, but 0.41% (95% CI, 0.31-0.53) for those younger than 65 years in a multicountry, patient level meta-analysis of 141,220 individuals. This yields a NNS of 69 to identify 1 new AF case for patients 65 years of age or older.[82] The choice of screening methodology, device, geographical region, or setting did not influence the AF detection rate. There is some debate regarding the utility of systematic screening compared with opportunistic case-finding. A performed in a United Kingdom primary care setting.[83],[84] In the first study 3001 patients older than 65 years of age were randomized to single time point nurse-led systematic screening vs prompted opportunistic case finding.[83] Despite substantially more patients who underwent assessment in the systematic screening arm (73% vs 29%) there was no significant difference in the identification of new AF cases (systematic 0.8% vs opportunistic 0.5%; odds ratio [OR], 1.72; 95% CI, 0.68-4.37).[83] The Screening for AF in the Elderly (SAFE) study was larger in scale (50 primary care practices, 14,802 patients), including 2 interventional groups (single time point systematic screening and opportunistic case finding) as well as a usual care control group. The SAFE study showed that both interventional groups identified significantly more new AF cases compared with usual care (OR, 1.6; 95% CI, 1.1-2.3; P ¼ 0.009); however, only opportunistic case finding was deemed to be cost-effective.[84] There is concern that single time point screening offers too finite a window of observation, which is particularly problematic in the context of intermittent AF. This limitation of single time point screening was shown in the STROKESTOP study, in which twice-daily intermittent period. The authors reported newly diagnosed AF in 3.0% of 75- and 76-year-old subjects, with repeated rhythm assessments leading to a fourfold increase in AF detection over single time point screening (initial single-lead ECG recording [SL-ECG]).[85] Although effective in AF case-finding, the utility of screening is dependent on population engagement. In the studies outlined above,81-85 only 50%-75% of eligible patients participated in systematic screening. Alternative screening environments might leverage existing health initiatives (eg, at time of influenza vaccination)[86] or other health care professionals (eg, pharmacy).[87],[88] An economic evaluation of an AF screening program requires consideration of several key factors: (1) participation rate; (2) rate of undiagnosed AF in a targeted population; (3) difference in AF detection between screening and usual care; (4) stroke risk in a targeted population; (5) stroke risk reduction and increase in bleeding risk from OAC; and (6) acceptable threshold for willingness to pay.[89] Intermittent opportunistic case finding was estimated to cost between 10V and 108V per patient (depending on the device, calculation method, and intensity of screening), which was lower than that of systematic screening.[81],[87],[90] A 2-part decision model to evaluate short- and long-term costs and quality-adjusted life years (QALYs) as part of the Program for the Identification of “Actionable” AF (PIAAF) in the pharmacy setting showed an incremental cost per QALY gained of CAD$7480 compared with no screening.[91] When different screening strategies were compared in a Canadian family practice study, screening with pulse check had the lowest expected costs (CAD$202) and screening with (SLECG) had the highest expected costs (CAD$222). The noscreening arm resulted in the lowest number of QALYs, whereas pulse check and SL-ECG resulted in the highest expected QALYs.[92] In a pooled analysis, the sensitivity of pulse palpation, blood pressure (BP) monitors, non-12-lead ECG, and smart phone applications were similar, but specificity was lower with pulse palpation. Because of the simplicity and ease of use, pulse palpation remains the cornerstone for AF detection. Downstream confirmatory testing with a 12-lead ECG should be pursued after pulse palpation, when screening is performed with non-rhythm-based devices (Table 5), or when the diagnosis of AF remains uncertain after rhythm acquisition.[92]–[94] Additional studies with robust methodology are needed to identify optimal screening strategies, screening tools, population, and settings with health outcomes for different health care systems. An approach to AF screening is shown in Figure 5.
Recommendation
6. We recommend that opportunistic screening for AF should be conducted in people 65 years of age and older at the time of medical encounters (Strong Recommendation; Low-Quality Evidence).
Practical Tip
Screening can be efficiently and cost effectively performed using opportunistic pulse checks during routine medical encounters; consideration can also be made to use rhythm-based devices (eg, SL-ECG rhythm device).
7. We recommend downstream confirmatory testing when AF is suspected but not documented, or when the documentation method does not include electrocardiographic rhythm acquisition (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
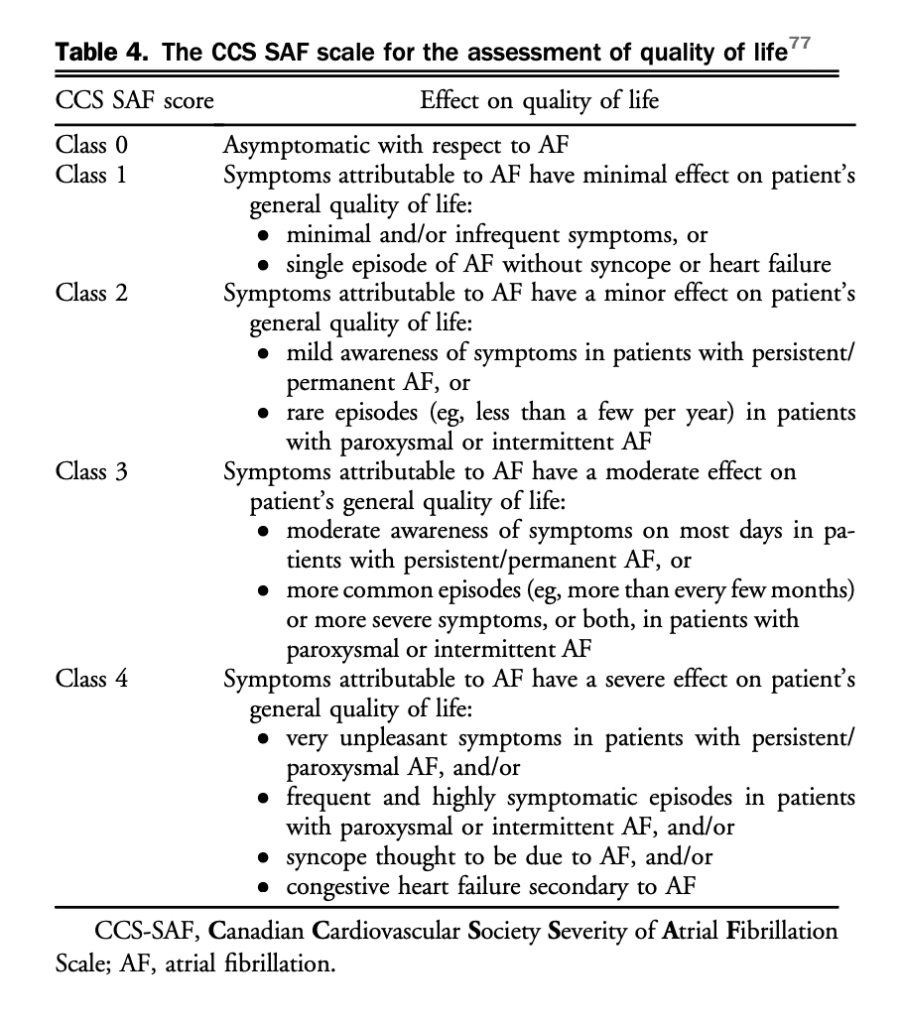
Confirmatory testing for AF is highly dependent on the type of AF. The effectiveness of the various AF screening methods depends on duration of monitoring (eg, single 12-lead ECG vs continuous monitoring). The use of new technologies for screening require validation before implementation.
5.2 Opportunistic AF detection in patients with a CIED
Advances in CIED (pacemaker or defibrillator) technology, which allow for long-term cardiac monitoring, have enabled detection of atrial high-rate episodes (AHREs) in patients with an atrial lead.[50],[95],[96] Because of the possibility of false positive results, a careful review of the electrograms should be performed.[97] Observational and registry data have shown at least a doubling of thromboembolic risk when AHREs are present compared with their absence.[50],[98]–[101] However, the adjusted stroke rates observed with AHREs appear to be lower than reported among patients with similar risk profiles and clinically apparent AF.[50],[99] Although evidence suggests a link between AHREs and elevated stroke risk, many uncertainties exist regarding relevant stroke risk factors, optimal atrial rate cut-off, minimum significant AF burden, and importantly, if OAC reduces AHRE-related stroke (see section 11.1).[50],[95],[96]
Recommendation
8. We recommend that AHREs be assessed at the time of CIED (loop recorders, pacemakers, or implanted cardioverter-defibrillators) interrogation (Strong Recommendation; Low-Quality Evidence).
5.3 AF detection after embolic stroke of undetermined source
AF accounts for a substantial proportion of acute ischemic strokes or transient ischemic attacks (TIAs) of known etiology, and might be responsible for a significant amount of strokes of undetermined etiology (embolic stroke of undetermined source [ESUS] or cryptogenic stroke).[102]–[104] In those with ESUS and no known history of AF, antiplatelet therapy remains the treatment of choice.[105] This was demonstrated in recent RCT, where empiric initiation of rivaroxaban 15mg daily was not superior to aspirin in preventing recurrent stroke, and was associated with an almost threefold higher risk of bleeding.[105],[106] However, it is known that antiplatelet therapy is inadequate for the treatment of AF-associated strokes (see section 8.2.1).[51],[107],[108] As such, ESUS patients require extensive AF screening to identify patients who would benefit from OAC for the secondary prevention of AFassociated thromboembolism.[51],[107],[108] Detection of AF is related to burden and improves with increasing intensity of monitoring.[109] In a systematic review of inpatient screening, the proportion of stroke patients newly diagnosed with AF on an admission 12-lead ECG was 7.7% (95% CI, 5.0-10.8), with an additional 7.0% (95% CI, 3.9- 10.8) diagnosed after inpatient cardiac telemetry, and an additional 4.5% (95% CI, 2.7-6.7) diagnosed after in-hospital Holter monitoring.[110] Prolonged postdischarge cardiac monitoring strategies (> 24 hours) have been evaluated in 3 RCTs for the purpose of AF detection after stroke.[111] A small United Kingdom study showed that a 7-day event monitoring uncovered new AF in 18% of patients compared with 2% in the control arm (P < 0.05%).[112] The 30-Day Cardiac Event Monitor Belt for Recording Atrial Fibrillation After a Cerebral Ischemic Event (EMBRACE) trial (N ¼ 572) showed a 30-day monitor detected AF in 16% of patients compared with 3.2% who underwent a 24-hour Holter monitoring within 90 days (P < 0.001; NNS ¼ 8), which led to nearly a doubling in the rate of OAC use.[72] The Cryptogenic Stroke and Underlying Atrial Fibrillation (CRYSTAL AF) trial (N ¼ 441) showed a sixfold higher rate of AF detection at 6 months using an implantable cardiac monitor compared with usual care.[73] At 3 years, the rate of AF detection was 30%, with most patients prescribed an OAC.[113] Prolonged rhythm monitoring was associated with a 51% reduction in the risk of recurrent stroke/TIA,[114] with the rates of recurrent cerebrovascular events or mortality being similar in patients who had known AF before their stroke and patients in whom AF was only diagnosed after their stroke.[115] Substudy analyses from the EMBRACE and CRYSTAL AF trials showed that prolonged rhythm monitoring was cost-effective for the prevention of recurrent stroke in patients with ESUS compared with usual care.[116],[117] Although there is no minimal AF burden threshold stipulated for which long-term OAC should be initiated for secondary prevention,[118] in the EMBRACE study a steep increase in OAC use was observed for patients found to have evidence of at least 30 seconds of AF on monitoring.[72] The authors acknowledged the lack of data to inform this matter but argue that the AF duration threshold at which to begin OAC might reasonably be lower for secondary stroke prevention compared with primary prevention. Further clinical trials are needed to determine the optimal duration and method of rhythm monitoring, the ideal population for extended rhythm monitoring, the minimum AF burden required for OAC initiation, and the efficacy of a prolonged monitoring strategy for the end point of recurrent stroke.
Recommendation
9. We recommend at least 24 hours of ambulatory ECG monitoring to identify AF in patients with nonlacunar ESUS (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation places relatively high value on the fact that stroke might be the first manifestation of previously undiagnosed AF.
10. We suggest additional monitoring for AF detection (eg, prolonged external loop recorder or implantable cardiac monitoring, where available) be performed for selected older patients with nonlacunar ESUS in whom AF is suspected but unproven (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
troke of unknown source. The main rationale is to improve the identification of patients who would have an evidence-based change in management aimed at preventing recurrent strokes (eg, switching from antiplatelet to OAC) if a clear diagnosis of AF is found. There are currently insufficient data to indicate what the minimum AF duration should be for OAC to be instituted and expert opinion varies widely, therefore treatment decisions should be individualized.
References
81. Mairesse GH, Moran P, Van Gelder IC, et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLAECE). Europace 2017;19:1589-623.
82. Lowres N, Olivier J, Chao TF, et al. Estimated stroke risk, yield, and number needed to screen for atrial fibrillation detected through single time screening: a multicountry patient-level meta-analysis of 141,220 screened individuals. PLoS Med 2019;16:e1002903.
83. Morgan S, Mant D. Randomised trial of two approaches to screening for atrial fibrillation in UK general practice. Br J Gen Pract 2002;52(373-4):377-80.
84. Hobbs FD, Fitzmaurice DA, Mant J, et al. A randomised controlled trial and cost-effectiveness study of systematic screening (targeted and total population screening) versus routine practice for the detection of atrial fibrillation in people aged 65 and over. The SAFE study. Health Technol Assess 2005;9. iii-iv, ix-x, 1-74.
85. Svennberg E, Engdahl J, Al-Khalili F, et al. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176-84.
86. Kaasenbrood F, Hollander M, Rutten FH, et al. Yield of screening for atrial fibrillation in primary care with a hand-held, single-lead electrocardiogram device during influenza vaccination. Europace 2016;18:1514-20.
87. Lowres N, Neubeck L, Salkeld G, et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. The SEARCH-AF study. Thromb Haemost 2014;111:1167-76.
88. Sandhu RK, Dolovich L, Deif B, et al. High prevalence of modifiable stroke risk factors identified in a pharmacy-based screening programme. Open Heart 2016;3:e000515.
89. Freedman B, Camm J, Calkins H, et al. Screening for atrial fibrillation: a report of the AF-SCREEN International Collaboration. Circulation 2017;135:1851-67.
90. Moran PS, Teljeur C, Ryan M, Smith SM. Systematic screening for the detection of atrial fibrillation. Cochrane Database Syst Rev 2016;6:CD009586.
91. Tarride JE, Dolovich L, Blackhouse G, et al. Screening for atrial fibrillation in Canadian pharmacies: an economic evaluation. CMAJ Open 2017;5:E653-61.
92. Tarride JE, Quinn FR, Blackhouse G, et al. Is screening for atrial fibrillation in Canadian family practices cost-effective in patients 65 years and older? Can J Cardiol 2018;34:1522-5.
93. Andrade JG, Godin R, Nault I. Large-scale implementation of a pragmatic atrial fibrillation screening program in Canadian community practice. Pacing Clin Electrophysiol 2020;43:768-9.
94. Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a Smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909-17.
95. Daoud EG, Glotzer TV, Wyse DG, et al. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli on the basis of stored device data: a subgroup analysis of TRENDS. Heart Rhythm 2011;8:1416-23.
96. Van Gelder IC, Healey JS, Crijns H, et al. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339-44.
97. Swiryn S, Orlov MV, Benditt DG, et al. Clinical implications of brief device-detected atrial tachyarrhythmias in a cardiac rhythm management device population: results from the Registry of Atrial Tachycardia and Atrial Fibrillation Episodes. Circulation 2016;134:1130-40.
98. Gorenek BC, Bax J, Boriani G, et al. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace 2017;19:1556-78.
99. Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation 2003;107:1614-9.
100. Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol 2009;2:474-80.
101. Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol 2005;46:1913-20.
102. Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population based study. Stroke 2005;36:1115-9.
103. Cotter PE, Martin PJ, Ring L, et al. Incidence of atrial fibrillation detected by implantable loop recorders in unexplained stroke. Neurology 2013;80:1546-50.
104. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429-38.
105. Johnston SC, Easton JD, Farrant M, et al; Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med 2018;379:215-25.
106. Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med 2018;378:2191-201.
107. Investigators AWGotA, Connolly S, Pogue J, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet 2006;367:1903-12.
108. Ntaios G, Papavasileiou V, Diener HC, Makaritsis K, Michel P. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke 2012;43:3298-304.
109. Andrade JG, Field T, Khairy P. Detection of occult atrial fibrillation in patients with embolic stroke of uncertain source: a work in progress. Front Physiol 2015;6:100.
110. Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015;14:377-87.
111. Kishore A, Vail A, Majid A, et al. Detection of atrial fibrillation after ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. Stroke 2014;45:520-6.
112. Higgins P, MacFarlane PW, Dawson J, et al. Noninvasive cardiac event monitoring to detect atrial fibrillation after ischemic stroke: a randomized, controlled trial. Stroke 2013;44:2525-31.
113. Brachmann J, Morillo CA, Sanna T, et al. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the Cryptogenic Stroke and Underlying Atrial Fibrillation trial. Circ Arrhythm Electrophysiol 2016;9:e003333.
114. Tsivgoulis G, Katsanos AH, Kohrmann M, et al. Duration of implantable cardiac monitoring and detection of atrial fibrillation in ischemic stroke patients: a systematic review and meta-analysis. J Stroke 2019;21:302-11.
115. Yang XM, Rao ZZ, Gu HQ, et al. Atrial fibrillation known before or detected after stroke share similar risk of ischemic stroke recurrence and death. Stroke 2019;50:1124-9.
116. Yong JH, Thavorn K, Hoch JS, et al. Potential cost-effectiveness of ambulatory cardiac rhythm monitoring after cryptogenic stroke. Stroke 2016;47:2380-5.
117. Diamantopoulos A, Sawyer LM, Lip GY, et al. Cost-effectiveness of an insertable cardiac monitor to detect atrial fibrillation in patients with cryptogenic stroke. Int J Stroke 2016;11:302-12.
118. Wein T, Lindsay MP, Cote R, et al. Canadian stroke best practice recommendations: secondary prevention of stroke, sixth edition practice guidelines, update 2017. Int J Stroke 2018;13:420-43.
