3. Pathophysiology and Risk Factors
AF is a complex and multifaceted condition ranging from an isolated electrophysiological disorder or, more commonly, a manifestation or consequence of other cardiac and noncardiac pathologies (Table 1, Fig. 2).[19] AF generally results from a combination of focal ectopic activity and reentry.[29],[60] Ectopic atrial foci arise from perturbations that cause cells to spontaneously depolarize, either secondary to enhanced automaticity or, more frequently, to triggered activity from after depolarizations. Discrete abnormalities in Ca2þ handling have been identified as centrally involved in after depolarization generation in paroxysmal and persistent AF, as well as postoperative AF (POAF).[61],[62] There is emerging evidence that inflammatory signalling plays a key role in promoting after depolarization generation, as well as other components of AF pathophysiology.[63] These repetitive rapid discharges predominantly originate from the pulmonary veins (PVs), which are a vulnerable region for triggered activity and micro reentry due to the shorter action potential duration, lower resting membrane potentials, and nonuniform myofibril arrangement.[64] When triggered, AF can be maintained by sustained rapid firing of focal impulses that disorganize into fibrillatory waves at their periphery or, in most cases, AF perpetuating reentry. Reentry requires specific conditions for initiation and maintenance. Although reentry is not sustained in the normal atrium, the presence of a vulnerable substrate can perpetuate AF through electrical heterogeneity (eg, regional differences in resting membrane potentials, refractory periods, action potential duration, and conduction velocities). In addition, conduction abnormalities can promote reentrant activity and stabilize reentrant circuits by creating functional barriers that allow recovery of tissue excitability. Structural abnormalities such as atrial fibrosis promote reentry through localized conduction slowing and structural conduction barriers, with atrial chamber dilatation promoting reentry through maintenance of the balance between rotor formation and rotor annihilation. Recently, there has been a renewed focus on the contribution of modifiable cardiovascular risk factors to the causation of AF, because an improved understanding of this relationship is key to providing effective personalized primary and secondary prevention measures.[29],[65],[66] Although the precise mechanistic links between risk factors and AF occurrence remain somewhat uncertain, information available from the literature provides many potential insights.[29] Hypertension, the most significant population attributable modifiable risk factor for AF, causes activation of the sympathetic and renin-angiotensin-aldosterone systems as well as structural and electrophysiological atrial remodelling that enhances AF susceptibility. Diabetes mellitus promotes AF via structural and autonomic remodelling. Tobacco use promotes AF through a combination of the direct effects of nicotine on the atrium (eg, altered atrial conduction and refractoriness) along with structural remodelling, inflammation, and oxidative stress. Alcohol, when consumed in excess, promotes AF through the induction of arrhythmia triggers (increased sympathetic activity/impairment of vagal tone) as well as atrial fibrosis (from the direct toxic effects of alcohol metabolites). Obesity promotes AF through weight-related structural (changes in atrial dimensions and interstitial fibrosis) and electrophysiological remodelling (conduction slowing and shortening of the effective refractory period), autonomic dysfunction, and inflammation. Obstructive sleep apnea (OSA) promotes AF acutely through strongly negative intrathoracic pressures leading to increased venous return (AF-promoting left atrial [LA] volume loading) and hypoxia-induced pulmonary vasoconstriction. Chronic OSA induces electrical and structural remodelling of the atria, autonomic dysregulation, oxidative stress, and inflammation. Regular exercise protects against AF by combating risk factors like obesity and metabolic syndrome but sustained intense exercise might promote AF occurrence (see section 11.3). The relationship between key risk factors and AF are outlined in Table 2.
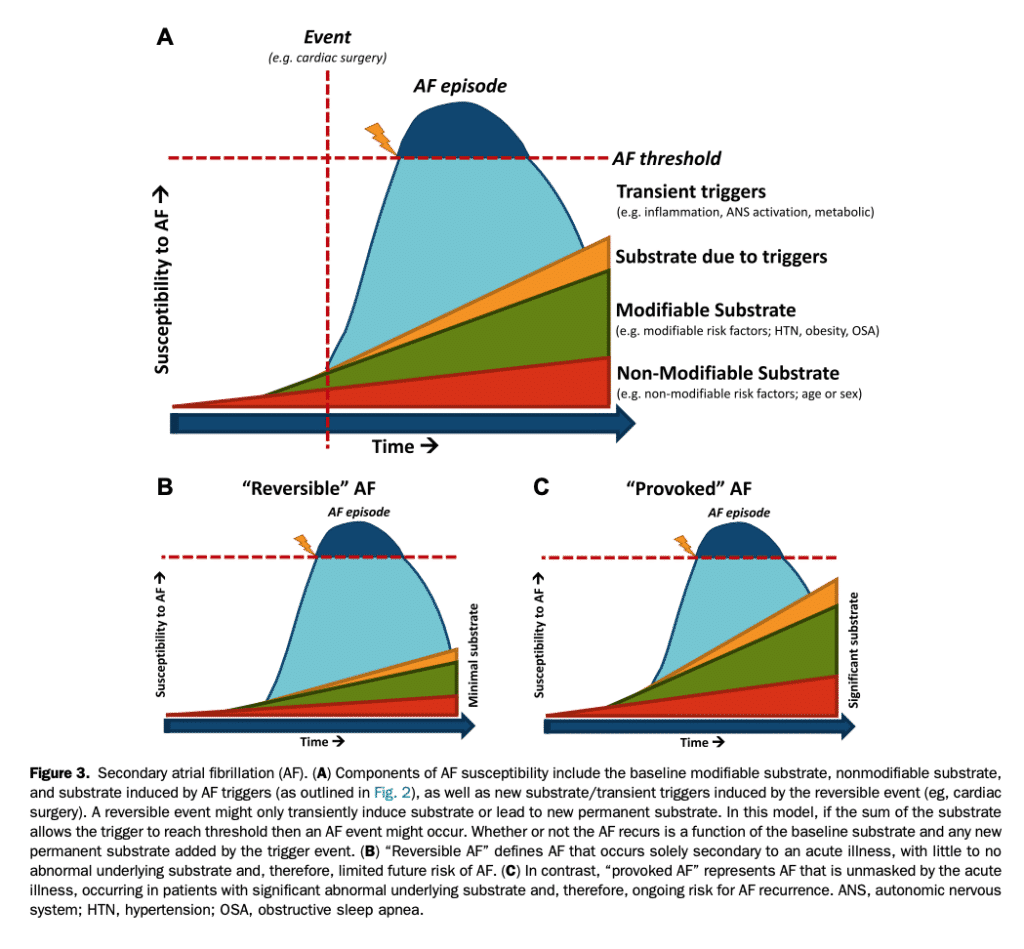
References
29. Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453-68.
30. Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population based estimates. Am J Cardiol 1998;82:2N-9N.
31. Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes 2012;5:85-93.
32. Kannel WB, Abbott RD, Savage DD, McNamara PM. Epidemiologic features of chronic atrial fibrillation: the Framingham study. N Engl J Med 1982;306:1018-22.
33. Furberg CD, Psaty BM, Manolio TA, et al. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am J Cardiol 1994;74:236-41.
34. Rietbrock S, Heeley E, Plumb J, van Staa T. Chronic atrial fibrillation: incidence, prevalence, and prediction of stroke using the Congestive heart failure, Hypertension, Age >75, Diabetes mellitus, and prior Stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J 2008;156:57-64.
35. Chiang CE, Naditch-Brule L, Murin J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol 2012;5:632-9.
36. Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370-5.
37. Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119-25.
38. Dorian P, Jung W, Newman D, et al. The impairment of health-related quality of life in patients with intermittent atrial fibrillation: implications for the assessment of investigational therapy. J Am Coll Cardiol 2000;36:1303-9.
39. Savelieva I, Paquette M, Dorian P, Luderitz B, Camm AJ. Quality of life in patients with silent atrial fibrillation. Heart 2001;85:216-7.
40. Singh SN, Tang XC, Singh BN, et al. Quality of life and exercise performance in patients in sinus rhythm versus persistent atrial fibrillation: a Veterans Affairs Cooperative Studies Program substudy. J Am Coll Cardiol 2006;48:721-30.
41. Thrall G, Lane D, Carroll D, Lip GY. Quality of life in patients with atrial fibrillation: a systematic review. Am J Med 2006;119. 448.e1-19.
42. Kang Y. Relation of atrial arrhythmia-related symptoms to health-related quality of life in patients with newly diagnosed atrial fibrillation: a community hospital-based cohort. Heart Lung 2006;35:170-7.
43. Wolf PA, Mitchell JB, Baker CS, Kannel WB, D’Agostino RB. Impact of atrial fibrillation on mortality, stroke, and medical costs. Arch Intern Med 1998;158:229-34.
44. Andersson T, Magnuson A, Bryngelsson IL, et al. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish nationwide long-term case-control study. Eur Heart J 2013;34:1061-7.
45. Benjamin EJ, Virani SS, Callaway CW, et al. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67-492.
46. Lee E, Choi EK, Han KD, et al. Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLoS One 2018;13:e0209687.
47. Petty GW, Brown RD Jr, Whisnant JP, et al. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke 2000;31:1062-8.
48. Bruggenjurgen B, Rossnagel K, Roll S, et al. The impact of atrial fibrillation on the cost of stroke: the Berlin Acute Stroke study. Value Health 2007;10:137-43.
49. Gladstone DJ, Bui E, Fang J, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke 2009;40:235-40.
50. Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120-9.
51. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007;146:857-67.
52. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955-62.
53. Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart 2004;90:286-92.
54. Humphries KH, Jackevicius C, Gong Y, et al. Population rates of hospitalization for atrial fibrillation/flutter in Canada. Can J Cardiol 2004;20:869-76.
55. Wu EQ, Birnbaum HG, Mareva M, et al. Economic burden and comorbidities of atrial fibrillation in a privately insured population. Curr Med Res Opin 2005;21:1693-9.
56. Lee WC, Lamas GA, Balu S, et al. Direct treatment cost of atrial fibrillation in the elderly American population: a Medicare perspective. J Med Econ 2008;11:281-98.
57. Kim MH, Lin J, Hussein M, Kreilick C, Battleman D. Cost of atrial fibrillation in United States managed care organizations. Adv Ther 2009;26:847-57.
58. O’Reilly DJ, Hopkins RB, Healey JS, et al. The burden of atrial fibrillation on the hospital sector in Canada. Can J Cardiol 2013;29:229-35.
59. Meyre P, Blum S, Berger S, et al. Risk of hospital admissions in patients with atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol 2019;35:1332-43.
60. Nattel S. New ideas about atrial fibrillation 50 years on. Nature 2002;415:219-26.
61. Voigt N, Heijman J, Wang Q, et al. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014;129:145-56.
62. Voigt N, Li N, Wang Q, et al. Enhanced sarcoplasmic reticulum Ca2þ leak and increased Naþ-Ca2þ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059-70.
63. Yao C, Veleva T, Scott L Jr, et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation 2018;138:2227-42.
64. Nishida K, Datino T, Macle L, Nattel S. Atrial fibrillation ablation: translating basic mechanistic insights to the patient. J Am Coll Cardiol 2014;64:823-31.
65. Pathak RK, Middeldorp ME, Lau DH, et al. Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64:2222-31.
66. Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation 2017;136:583-96.
