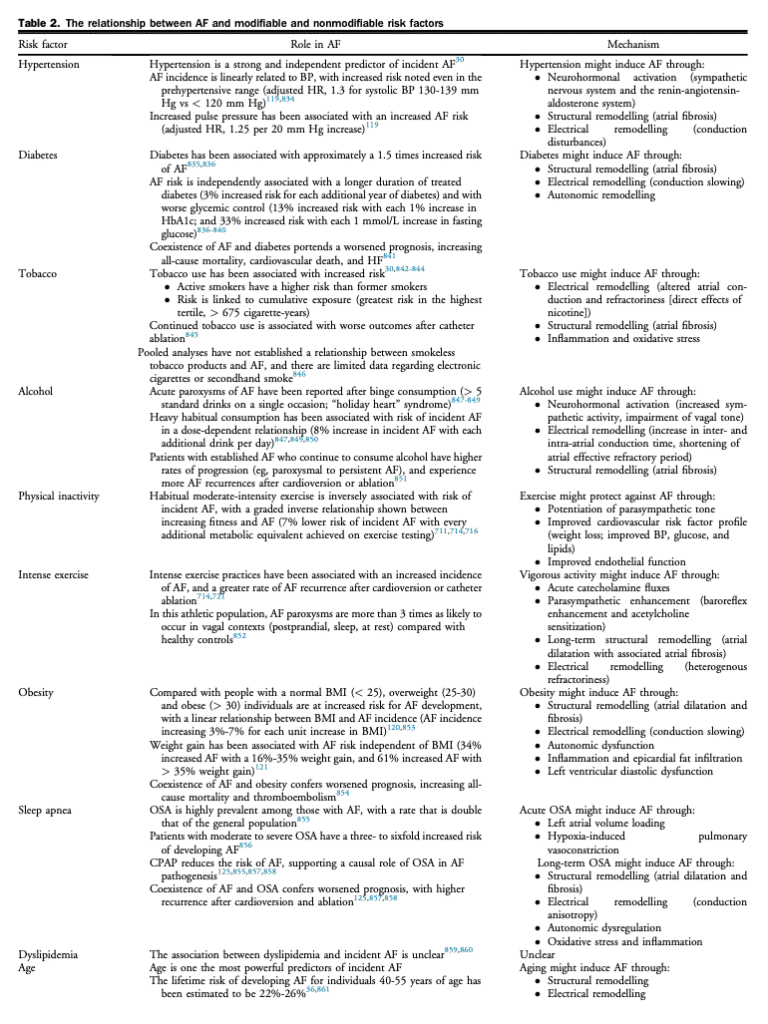
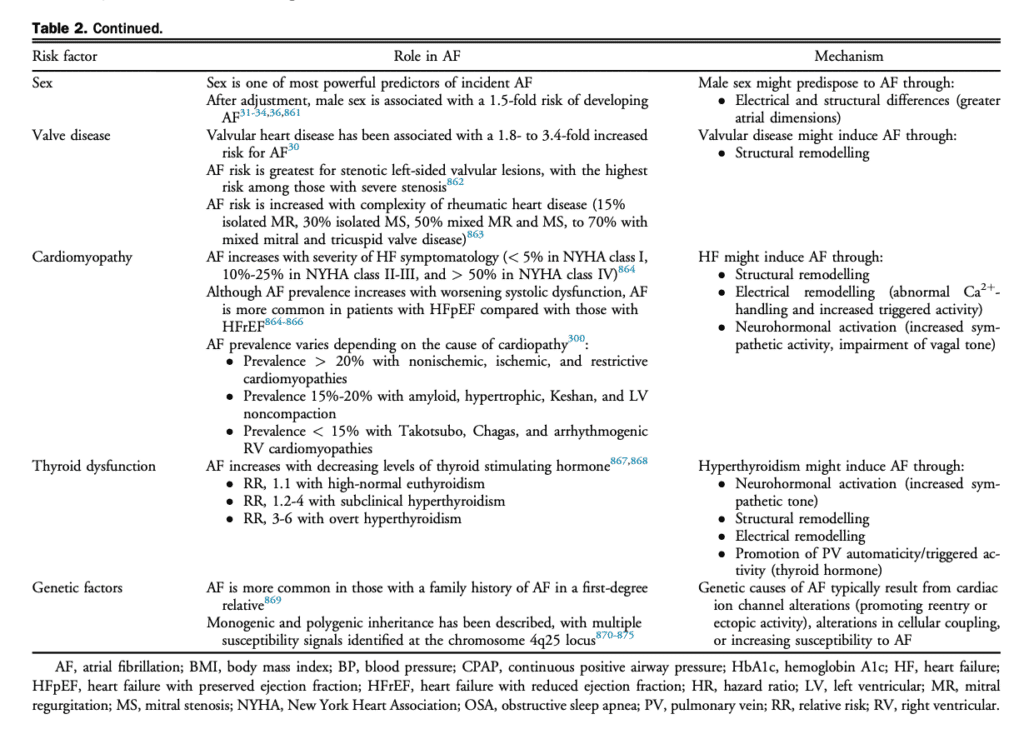
4. Clinical Evaluation
The purpose of the initial evaluation of a patient with AF is to establish the magnitude and severity of symptoms attributable to AF, identify the underlying etiology and precipitants of AF, establish prognosis, and develop a therapeutic strategy for symptom relief and morbidity mitigation (Fig. 4).
4.1 AF history
A comprehensive AF history should include the date of first symptomatic attack as well as the date of first ECG documentation. For patients in AF at the time of assessment, the timing of onset for the current AF episode should be determined. The duration and frequency of episodes should be used to establish the predominant pattern of AF (paroxysmal vs persistent; see section 1). Of note, in some cases the symptom evaluation might be insufficient for the determination of AF pattern and additional monitoring might be required.[12],[13] The presence and nature of AF-related symptoms, their severity, and their effect on QOL should be determined (see section 4.3). Symptoms might be absent or manifest as palpitations, dyspnea, dizziness, weakness, fatigue, or chest pain.[67] In addition, it is important to elicit any history of regular palpitations because any supraventricular tachycardia (SVT) can lead to the development of AF, and ablation of the SVT might eliminate or substantially reduce the likelihood of recurrent AF (see section 11.7).[68],[69] Symptoms at the termination of AF episodes, such as presyncope or syncope, should be determined because significant sinus pauses might limit the use of rate- or rhythm-controlling medications and might require the use of permanent pacing or prompt early ablation. Precipitating factors (“triggers for AF episodes”), reversible causes, and coexisting cardiovascular risk conditions should be determined. These include modifiable cardiovascular risk factors and comorbid conditions, which if treated, might reduce or eliminate AF recurrence and improve the overall outcome of the patient, independent of AF (see section 6).[32],[70] Past evaluations and treatments should be explored, including a record of all previous pharmacologic and nonpharmacologic AF interventions (eg, cardioversion and catheter ablation). AF-related health care utilization should be documented, including a record of emergency department (ED) visits, hospital admissions, and cardioversions. Risk factors for stroke (see section 8.1) and bleeding (see section 8.5.1) should be elicited. The precise frequency, duration, and intensity of sports participation (current and previous) needs to be assessed carefully for all AF patients (see section 11.3). In addition, the evaluation should include: a comprehensive review of all prescription, over the counter, and nonprescription medications; a social history with a focus on alcohol, tobacco, and recreational drug intake; and a family history of cardiac dysrhythmia or relevant risk conditions.

4.2 Investigations
In addition to a comprehensive physical examination several routine investigations are warranted for all patients who present with AF (Fig. 4):
AF must be electrocardiographically documented, because the perception of “irregularly irregular” palpitations might be the result of a variety of arrhythmias, including atrial tachycardia, atrial flutter (AFL), premature atrial and/or ventricular contractions, or nonarrhythmic causes.
An ECG is useful in AF and sinus rhythm to identify LA enlargement, left ventricular (LV) hypertrophy (LVH), preexcitation, conduction disease, or evidence of MI.
A transthoracic echocardiogram should also be performed in all patients to identify LVH or systolic dysfunction, significant valvular or congenital heart disease (CHD), LA enlargement, and rarely, complications such as intracardiac thrombus. LA dimension provides important information about the likelihood of AF recurrence or progression to persistent AF, which can help guide therapeutic decision-making.
Transesophageal echocardiography (TEE) might be indicated for more detailed evaluation of valvular or CHD, or for the exclusion of LA appendage (LAA) thrombus.
Routine blood biochemistry should be obtained at the time of the initial AF evaluation. A complete blood count and coagulation studies will inform decisions about the use of antithrombotic medications. Serum electrolytes and creatinine should be measured, and renal function determined to guide drug dosing (eg, creatinine clearance [CrCl], see section 8.3.1). Liver function should be assessed before the prescription of potentially hepatotoxic medications, such as amiodarone. Lipid profile, hemoglobin A1c, and fasting blood sugar are recommended in most patients as part of a comprehensive cardiovascular risk assessment. Thyroid function should be assessed because hyperthyroidism remains an important treatable cause of AF.[71] In select cases N-terminal pro-B-type natriuretic peptide (NT pro-BNP) and inflammatory biomarkers might aid in patient management.
Ambulatory ECG monitoring might aid in the documentation of AF, the identification of other arrhythmias, the assessment of ventricular rate control, and to correlate patient symptoms with heart rhythm or heart rate. In selected patients long-term monitoring using external loop recorders, wearable patch monitors, and cardiac implantable electronic devices (CIEDs) might be useful.[12],[13],[72],[73] In addition, consumer-facing devices (eg, handheld or wearable ECG devices) may be used to document heart rate or cardiac rhythm, potentially providing symptom-rhythm correlation and a measure of AF burden.
Sleep study or overnight oximetry should be performed in most patients because typical symptoms are less prevalent and screening questionnaires are less accurate in the AF population.
Exercise testing can be used to supplement ambulatory monitoring in certain patients with exerciserelated symptoms and might be helpful to exclude significant ischemia before class Ic antiarrhythmic drug prescription.
Invasive electrophysiological studies may be considered in patients who are candidates for catheter ablation of AF or with suspected SVT, which could be triggering AF (see sections 9.4 and 11.7).
4.3 Evaluation of the effect of AF on well-being, symptoms, and QOL
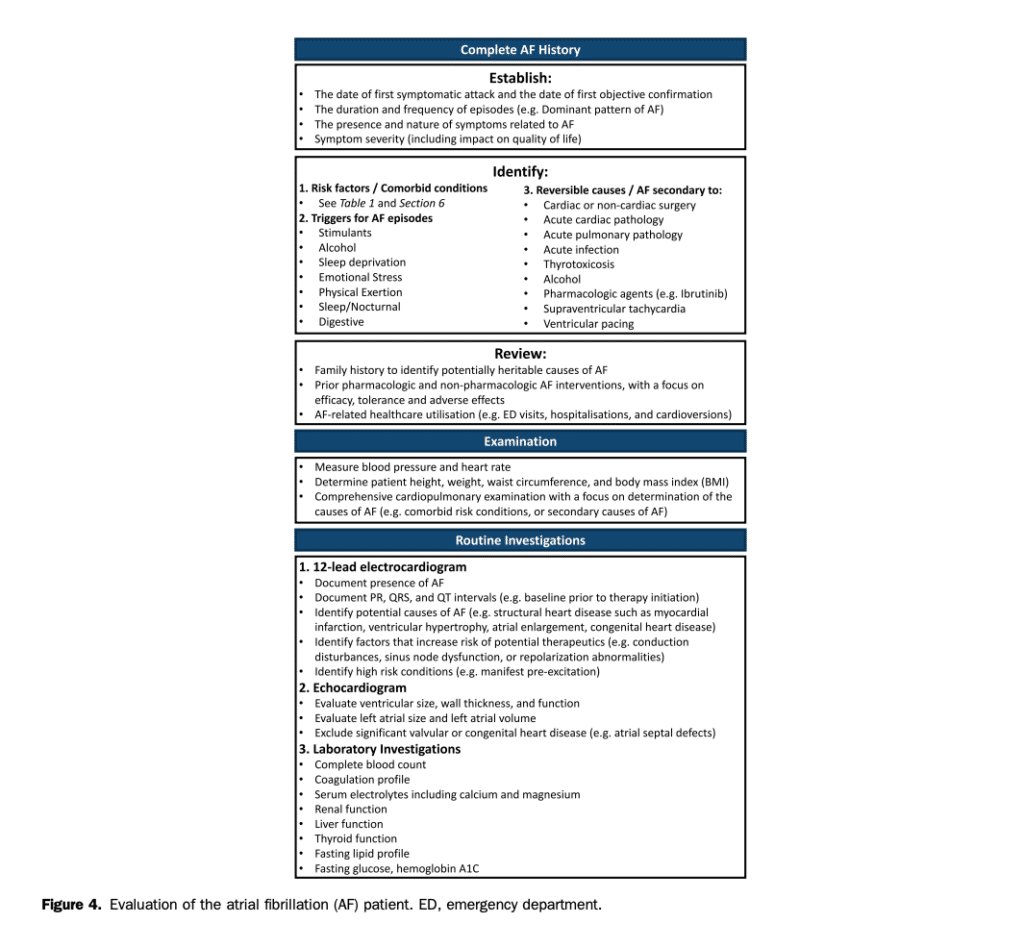
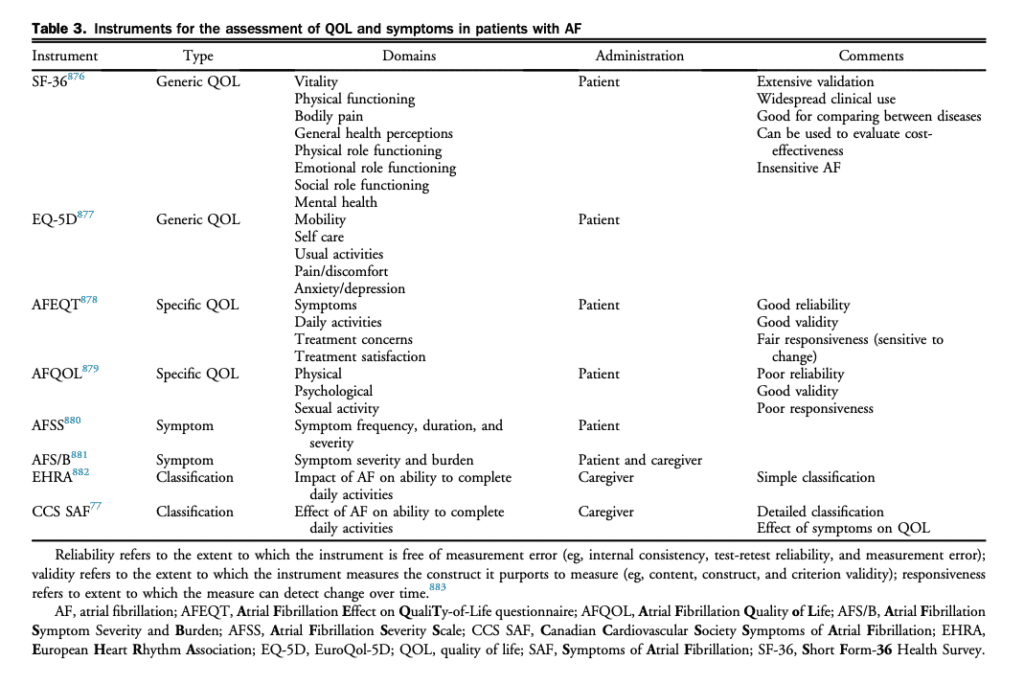
Traditional rhythm-based outcome parameters, such as freedom from AF recurrence, are insufficient to evaluate the clinical effect of AF.[74],[75] Although rarely lifethreatening, AF causes a greater degree of impairment of QOL than is generally appreciated. AF can cause moderate and sometimes severe distress, and substantially alter everyday functioning.[76] Impaired QOL is primarily the result of AF associated symptoms but can be influenced by AF therapies, illness perceptions, and patient factors such as anxiety or depression.[74],[75] A consistent and standardized assessment of the effect of AF on HRQOL is recommended to evaluate the clinical effect of AF and quantitatively assess the changes in well-being resulting from therapeutic interventions. Specifically, multidimensional HRQOL instruments can be used to determine if an intervention had a beneficial effect across all domains concurrently or if a benefit in one domain (eg, physical health) was offset by a negative effect in another (eg, mental health). As such, the assessment of patient reported outcomes with validated multidimensional instruments offers a relevant and complementary means to evaluate the consequences of AF and the effect of therapeutic interventions on patients’ functional status and health. To date, a large number of instruments have been used to evaluate HRQOL (Table 3). In broad terms, these instruments can be dichotomized into generic and diseasespecific questionnaires. Generic instruments, such as the EuroQol-5D (EQ-5D) and Short Form-36 Health Survey (SF-36), are used to assess valuations of health and functioning across a predefined set of health related domains. Generic instruments have the advantages of extensive validation across a wide range of populations and health conditions but lack precision for assessing the effect of AF. Disease-specific instruments include symptomspecific scales (eg, the University of Toronto Atrial Fibrillation Severity Scale [AFSS]), and AF-specific QOL symptom scales (eg, the Atrial Fibrillation Effect on Quality-of-Life [AFEQT] questionnaire). These instruments do not provide for the ability to compare between disease states (eg, the HRQOL of AF patients relative to HF patients) but are more sensitive to changes in AF patients’ health status (spontaneous or as a result of intervention). The Severity of Atrial Fibrillation (SAF; Table 4) is a semiquantitative scale ranging from 0 (no effect of AF or its treatment on overall QOL and patient functioning) to 4 (resulting in a severe impairment of functioning and overall QOL).[77] A multicentre Canadian study has shown that the results of this scale correlate well with previously validated symptom scores and generic measures of QOL and that it can be easily applied by a variety of caregivers at the bedside.[67],[78] Equally important is consideration of advanced age, frailty, cognitive impairment, and mood disorders. Identifying these factors might substantially affect anticipated QOL benefits of various AF therapies and can also help to identify potential challenges in adherence to therapy and self-care (see section 7.2).[79],[80]
Recommendation
1. We recommend that the initial evaluation of a patient with newly diagnosed AF include: a complete history and physical examination, a 12-lead ECG, a transthoracic echocardiogram, and basic laboratory investigations as outlined in Figure 4 (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation places a high value on a comprehensive evaluation of patients with AF and a lower value on initial costs to the health care system.
2. This recommendation places a high value on a comprehensive evaluation of patients with AF and a lower value on initial costs to the health care system.
Values and Preferences
This recommendation places a high value on evidence supporting a reduction in AF recurrence with treatment of the underlying condition or reversible precipitating event, such as infection, surgery, or thyroid disease.
Practical Tip
Elimination of the precipitating event does not completely eliminate the possibility of AF recurrence, meaning patients with secondary AF should be regularly screened for arrhythmia recurrence (see section 11.5.3).
3. We suggest that all individuals with AF should be assessed for their sports and exercise history, with special attention to frequency, duration, intensity, and type of sport (Weak Recommendation; Low-Quality Evidence).
4. We recommend that patient-reported AF-related symptoms and QOL be assessed with validated instruments as part of the longitudinal management of patients with AF (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation places a high value on the recognition that symptom control has a powerful influence on disability and health care resource utilization. The assessment of patient-reported outcomes with validated multidimensional instruments offers a relevant and complementary means to evaluate the effect of therapeutic interventions.
Practical Tip
The caregiver-administered CCS SAF scale can be used to assess functional status and the symptomatic effect of AF, and the self-administered disease-specific AFEQT questionnaire can be used to assess the effect of AF on QOL. Consideration can also be made to assess QOL using generic instruments such as the EQ5D questionnaire.
5. We recommend that patients with AF should be assessed for multimorbidity, frailty, cognitive impairment, dementia, and depression (Strong Recommendation; Low-Quality Evidence).
Practical Tip
In this population a structured, integrated, multidisciplinary, patient-focused approach to care can be particularly beneficial because these factors might have an effect on treatment decisions and might improve adherence to therapy and self-care.
References
12. Charitos EI, Purerfellner H, Glotzer TV, Ziegler PD. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J Am Coll Cardiol 2014;63:2840-8.
13. Andrade JG, Yao RRJ, Deyell MW, et al. Clinical assessment of AF pattern is poorly correlated with AF burden and post ablation outcomes: a CIRCA-DOSE sub-study. J Electrocardiol 2020;60:159-64.
67. Dorian P, Guerra PG, Kerr CR, et al. Validation of a new simple scale to measure symptoms in atrial fibrillation: the Canadian Cardiovascular Society Severity in Atrial Fibrillation scale. Circ Arrhythm Electrophysiol 2009;2:218-24.
68. Haissaguerre M, Fischer B, Labbe T, et al. Frequency of recurrent atrial fibrillation after catheter ablation of overt accessory pathways. Am J Cardiol 1992;69:493-7.
69. Brembilla-Perrot B, Olivier A, Villemin T, et al. Prediction of atrial fibrillation in patients with supraventricular tachyarrhythmias treated with catheter ablation or not. Classical scores are not useful. Int J Cardiol 2016;220:102-6.
70. Wolf PA, Dawber TR, Thomas HE Jr, Kannel WB. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke: the Framingham study. Neurology 1978;28:973-7.
71. Kim DD, Young S, Cutfield R. A survey of thyroid function test abnormalities in patients presenting with atrial fibrillation and flutter to a New Zealand district hospital. N Z Med J 2008;121:82-6.
72. Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467-77.
73. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478-86.
74. Hindricks G, Piorkowski C, Tanner H, et al. Perception of atrial fibrillation before and after radiofrequency catheter ablation: relevance of asymptomatic arrhythmia recurrence. Circulation 2005;112:307-13.
75. Verma A, Champagne J, Sapp J, et al. Discerning the incidence of symptomatic and asymptomatic episodes of atrial fibrillation before and after catheter ablation (DISCERN AF): a prospective, multicenter study. JAMA Intern Med 2013;173:149-56.
76. Walters TE, Wick K, Tan G, et al. Psychological distress and suicidalideation in patients with atrial fibrillation: prevalence and response to management strategy. J Am Heart Assoc 2018;7:e005502.
77. Dorian P, Cvitkovic SS, Kerr CR, et al. A novel, simple scale for assessing the symptom severity of atrial fibrillation at the bedside: the CCS-SAF scale. Can J Cardiol 2006;22:383-6.
78. Reynolds MR, Ellis E, Zimetbaum P. Quality of life in atrial fibrillation: measurement tools and impact of interventions. J Cardiovasc Electrophysiol 2008;19:762-8.
79. Miyazaki M, Nakashima A, Nakamura Y, et al. Association between medication adherence and illness perceptions in atrial fibrillation patients treated with direct oral anticoagulants: an observational crosssectional pilot study. PLoS One 2018;13:e0204814.
80. Miura K, Ikemura N, Kimura T, et al. Treatment strategies and subsequent changes in the patient-reported quality-of-life among elderly patients with atrial fibrillation. Am Heart J 2020;222:83-92.
