3. Revascularization Procedures for PAD
3.1 Preoperative assessment and risk stratification
3.1.1 Guideline rationale, development, and over-riding principles
Since the publication of the 2017 CCS guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery,[90] new evidence has emerged for evaluating clinical risk indices specific to patients who undergo peripheral vascular surgery. The scope of this section includes the evaluation of available evidence for preoperative assessment and risk stratification of patients who undergo nonurgent vascular surgery for PAD along the following themes: clinical risk indices, cardiac biomarkers, and noninvasive testing.
The recommendations pertain to adult patients, 18 years of age or older, who undergo elective open or endovascular arterial revascularization procedures of the lower extremities. Patients who require emergency lower extremity arterial revascularization should not have their surgery delayed by a preoperative risk assessment. The risks to life or limb that could result from a delay in surgery are often greater than the risks of not assessing and subsequently optimizing a patient’s preoperative cardiac status. However, operative risks must be explained to the patient and their family. Conversely, patients who undergo scheduled, nonemergency lower extremity arterial revascularization surgery should undergo cardiac risk assessment. Physicians or surgeons with training and competency in cardiac risk assessment should perform preoperative assessments.
3.1.2 Risk tools
PICO 3.1a: Which risk tool(s) best predict(s) perioperative CV risk and mortality?
Individuals who undergo surgery for PAD have a higher preoperative CV risk compared with other noncardiac surgeries. Various tools have been developed and evaluated to predict the perioperative risk of vascular surgery patients. Tools that included preoperative components only were considered. The following were examined: the Vascular Study Group of New England Cardiac Risk Index (VSG-CRI), the Vascular Quality Initiative Cardiac Risk Index (VQI-CRI), and frailty scores (single domain and multidomain). The evidence for Revised Cardiac Risk Index (RCRI) was also reassessed with a focus on PAD to determine whether its predictive value differs compared with a heterogenous pool of noncardiac surgeries. The summary of the findings and GRADE Evidence Profile for the 3 clinical risk scores are available on ccs.ca.
RCRI. For vascular surgery, RCRI consistently under-estimated the risk of MACE with poor discriminatory ability with the median area under the curve of 0.64 (range, 0.58-0.70; see ccs.ca for table). A number of serious limitations were identified in these studies, and the resulting bias would have been expected to falsely amplify the ability of RCRI to predict MACE. However, despite these limitations, RCRI still had poor prediction even in the largest study with subgroup analysis of lower extremity bypass.
VSG-CRI risk tool. This is a simple scoring algorithm that was derived from a vascular surgery cohort; the Vascular Study Group of New England. It was developed by assigning weighted points to each statistically significant predictor from a multivariate analysis and stratified patients prepared to undergo vascular surgery into 1 of 6 risk categories (0-3, 4, 5, 6, 7, 8) and predicted risk of MACE (2.2%, 3.5%, 6%, 6.6%, 8.9%, 14.3%), respectively. MACE were defined as in-hospital MI, clinically significant arrhythmia, or congestive heart failure (Fig. 5).
INFRA VQI-CRI risk score. This risk score was developed from the Vascular Quality Initiative, the largest vascular surgery-specific database assembled to date representing data from the United States and Canada.[92] An all-procedure model and procedure-specific models were developed (eg, Infrainguinal [INFRA] VQI-CRI for infrainguinal bypass procedures) to predict postoperative MI. The risk tools are freely available online and can be downloaded for offline use through the app, Calculate by QxMD, at https://qxmd.com/calculate/calculator_323/vascular-quality-initiative-vqi-cardiac-risk-index-cri-infra-inguinal-bypass.
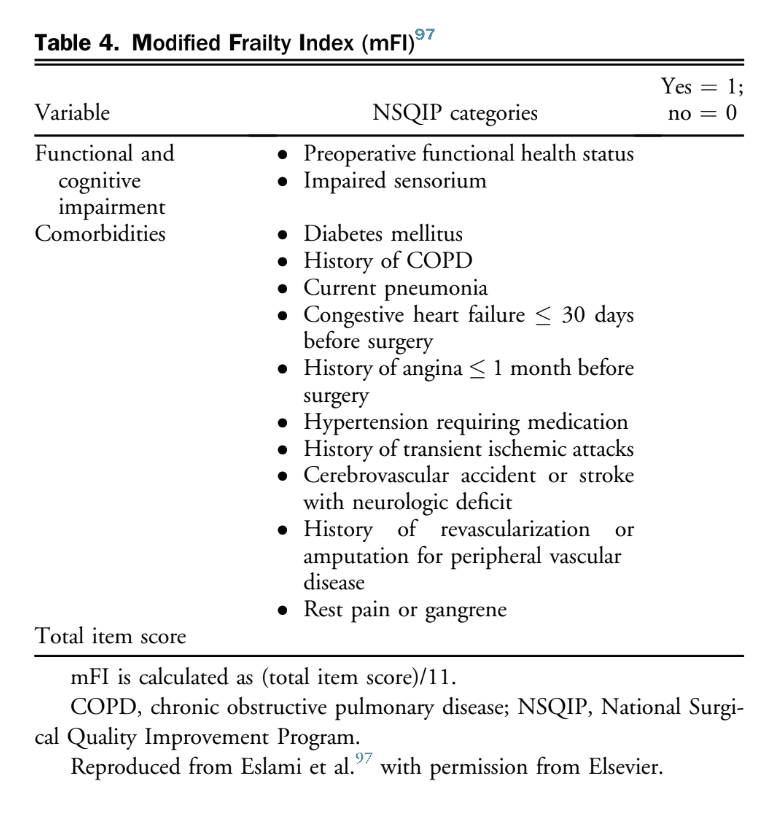
Frailty scores. The following tools were examined: single-domain (modified Frailty Index [mFI], and the Groningen Frailty Indicator [GFI]).[93]–[96] Functional dependency is classified as independent if no assistance is needed and dependent if there is some or full dependency for functions. The mFI comprises 11 items, of which 9 are comorbidities, along with measures of functional dependency and cognition variables.
The score is calculated by adding 1 point for the presence of each variable, then dividing the sum by 11 (Table 4). Frailty is defined as mFI 0.25. The GFI includes 16 items organized into 8 different groups. This tool would require further validation studies in peripheral arterial surgery before developing a recommendation regarding its use.
Other prediction tools such as Vascular-Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity (V-POSSUM) (with only preoperative components), Preoperative Score to Predict Postoperative Mortality (POSPOM), and modified RCRI, were considered but studies of those prediction tools were small, of low quality, and lacked validation using large databases.[98]–[100]
Recommendation
35. We recommend against using RCRI for preoperative assessment of cardiac risk in peripheral arterial surgery (Strong Recommendation; Moderate-Quality Evidence). Alternate options include the VSG-CRI and INFRA VQI-CRI (see the following recommendations).
Recommendations
36. When evaluating cardiac risk:
a) We suggest that clinicians use the VSG-CRI to predict MACE in patients who undergo peripheral vascular surgery (Weak Recommendation; Low-Quality Evidence); or
b) We suggest that clinicians use the INFRA VQI-CRI score to predict postoperative MI in patients who receive peripheral vascular surgery (Weak Recommendation; Low-Quality Evidence).
37. We recommend against using the RCRI for preoperative assessment of 30-day mortality in peripheral arterial surgery (Strong Recommendation; Low-Quality Evidence) and alternatively suggest using frailty scores (functional dependency or mFI) over other available risk prediction scores for 30-day mortality assessment (Weak Recommendation; Low-Quality Evidence for functional dependency; Moderate-Quality Evidence for mFI).
Values and Preferences
Higher preference and value are given to tools that are readily available and are quick to complete in the context of preoperative care clinics. Preference was also given to tools derived directly from the population of interest (ie, infrainguinal revascularization) because of data that support more reliable prediction than those developed from heterogenous surgical populations.
Practical Tip
Although a single risk tool to assess the risk of cardiac events and 30-day mortality would be clinically useful, such a tool was not identified in the current literature search. For 30-day mortality risk assessment, frailty scores are suggested, and for cardiac risk assessment the VSG-CRI or INFRA VQI CRI are suggested. An online and free calculator is available to assist clinicians with using the INFRA VQI-CRI risk score for postoperative MI prediction.
3.1.3 Cardiac biomarkers
PICO 3.1b: What is the role of preoperative cardiac biomarkers in risk prediction?
Cardiac biomarkers have been shown to help predict MACE in vascular surgery.[101] Brain natriuretic peptide (BNP) and N-terminal fragment pro hormone BNP (NT-proBNP) have shown some promising results (see ccs.ca for evidence tables). The recommendations are largely consistent with the CCS 2017 perioperative guidelines for non-cardiac surgery with a suggested threshold of preoperative NT-proBNP 300 mg/L or BNP 92 mg/L associated with an estimate risk of 21.8% (95% CI, 19.0%-24.8%) for composite of death and nonfatal MI at 30-days postoperatively.[101]
Recommendation
38. We suggest that clinicians consider measuring BNP or NT-proBNP before peripheral arterial surgery to enhance perioperative cardiac risk estimation (Weak Recommendation; Low-Quality Evidence).
Values and Preferences
Cost, accessibility, and reliability of the thresholds were considered for this recommendation. BNP and NT-proBNP are inexpensive and widely available tests. Patients might benefit from further stratification using biomarkers to guide perioperative care and surveillance of cardiac events preoperatively.
Practical Tip
Clinicians might consider clinical monitoring postoperatively in individuals with an elevated perioperative troponin level, preoperative NT-proBNP 300 mg/L or BNP 92 mg/L because of the significantly increased risk in these patient groups.[101] Point of care testing is an alternative for obtaining BNP/NT-proBNP levels within minutes in the preoperative setting or where testing is not available through core laboratories.
3.1.4 Noninvasive cardiac testing
PICO 3.1c: What is the role of preoperative noninvasive cardiac testing?
The literature search did not identify new, robust studies that examined the utility of noninvasive cardiac testing for peripheral arterial surgery.
Practical Tip
In the absence of new and robust evidence to inform noninvasive testing for peripheral arterial surgery, we suggest referring to the CCS 2017 guidelines for noncardiac surgery.[82] It is essential to continue to apply clinical judgement and current standards of practice for ordering noninvasive testing preoperatively while keeping in mind that the general risk of noncardiac surgery does not reflect the relatively increased cardiac and mortality risk of patients who undergo peripheral arterial surgery.
3.2 Indications for revascularization
PICO 3.2: What are the indications for revascularization procedures in patients with PAD on the basis of their clinical presentation (intermittent claudication, critical limb ischemia, acute limb ischemia, or asymptomatic)?
3.2.1 Intermittent claudication
Symptoms of claudication can manifest as pain, weakness, or numbness in the lower extremity induced with activity, usually walking. Common muscle groups involved include the calf, thigh, or buttock regions depending on the level of atherosclerotic disease (see section 1.1). The natural history of intermittent claudication generally includes a 2%-3% annual risk of progression to chronic limb-threatening ischemia after the first year of diagnosis.[102]–[104] In addition, the annual risk of amputation is 1% in these patients.[102],[103] Although the management of intermittent claudication is predominantly risk factor modification,[105],[106] revascularization can be considered in patients who continue to have lifestyle-limiting symptoms despite best medical management with an acceptable risk profile, reasonable expectation for functional improvement and life expectancy, and in whom a trial of nonoperative therapy with an exercise program on the basis of data from RCTs has failed.
Recommendation
39. We suggest that revascularization may be considered in patients with intermittent claudication affecting vocational, recreational, or daily living activities who have an acceptable risk profile, reasonable expectation for function and life expectancy, and in whom a trial of nonoperative therapy with an exercise program and optimal medical therapy has failed (Weak Recommendation; Moderate-Quality Evidence).
Recommendation
40. We recommend that the choice of revascularization procedure for intermittent claudication should be individualized, have the expectation of low perioperative morbidity, and have a reasonable likelihood of providing sustained symptomatic benefit (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation emphasizes that early intervention on lesions in individuals with claudication has not been shown to favourably alter the patient’s natural history or propensity to develop chronic limb-threatening ischemia.
3.2.2 Chronic limb-threatening ischemia
Unlike claudication, chronic limb-threatening ischemia has a worse natural history with increased propensity for tissue and limb loss.[107] Chronic limb-threatening ischemia is the most advanced form of PAD and patients with chronic limb-threatening ischemia often present with signs of severe arterial insufficiency such as ischemic rest pain, tissue loss, or gangrene.[108] Despite advances in pharmacological risk reduction therapy for PAD over the past 2 decades,[109],[110] chronic limb-threatening ischemia patients continue to have high mortality (22% over 1 year) and major amputation (22% over 1 year) rates without revascularization.[111] As such, in addition to CV risk reduction (see sections 2.1-2.6), prompt revascularization is the cornerstone of management for this condition.[112]
Patients with chronic limb-threatening ischemia should be urgently assessed by vascular specialists who have expertise in endovascular, open, and hybrid surgical techniques for peripheral revascularization. A thoughtful approach to selecting the type of revascularization procedure involves a timely assessment of patient, disease, and procedural factors (see section 3.4).
In addition to revascularization, special attention is required for the management of any associated tissue loss, gangrene, or infection. In patients who present with a deep foot infection or wet gangrene, urgent foot debridement or minor amputation should be considered before revascularization. Close follow-up, appropriate wound care, and frequent reassessment for further debridement are essential in ensuring ischemic and diabetic foot wounds heal after revascularization.
Some chronic limb-threatening ischemia patients might benefit from primary major amputation over a revascularization attempt, such as those with advanced nonreconstructable disease, nonambulatory status, severe sepsis due to progressive limb infection, and those who are unfit for revascularization (often nursing home or bedridden patients).
Recommendation
41. We recommend that all patients with chronic limb-threatening ischemia should be urgently referred to vascular specialists for consideration of revascularization (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation places a high value on timely evaluation of chronic limb-threatening ischemia patients by vascular specialists to decrease pain, improve wound healing, and ultimately prevent limb loss.
Recommendations
42. We recommend that in patients with chronic limb-threatening ischemia, endovascular, open, or hybrid revascularization should be considered on the basis of the anatomical pattern of disease, degree of ischemia, expected durability of the procedure, perioperative risk, and patient life expectancy (Strong Recommendation; Low-Quality Evidence).
43. We recommend wound debridement and/or minor amputation simultaneously with revascularization or in a staged manner depending on the degree of tissue loss, gangrene, and/or infection (Strong Recommendation; Low-Quality Evidence).
Practical Tip
Urgent debridement or minor amputation before revascularization is recommended if deep foot infection or wet gangrene is present.
Recommendation
44. We recommend primary major amputation in chronic limb-threatening ischemia patients with non-reconstructible disease, nonsalvageable limb, non-ambulatory status, severe sepsis, or for palliation for those with a short life expectancy and who are unfit for revascularization (Strong Recommendation; Low- Quality Evidence).
Values and Preferences
This recommendation places a high value on primary amputation for chronic limb-threatening ischemia patients who are unlikely to benefit from revascularization.
3.2.3 Acute limb ischemia
Acute limb ischemia, which occurs because of a sudden decrease in limb perfusion (usually within minutes to hours), is distinct from chronic limb-threatening ischemia because there is inadequate collateral circulation present to maintain imminent limb viability. Patients with acute limb ischemia have a poor prognosis, because delays in diagnosis or treatments are associated with high rates of major amputation and mortality.[113] Revascularization options include percutaneous catheter-directed thrombolytic therapy or mechanical thrombus extraction or aspiration, surgical thrombectomy, and surgical reconstruction. Treatment strategies are usually individualized and dependent on the degree and duration of ischemia present, viability of the limb, anatomical factors, patient risk profile, and availably of surgical and endovascular expertise.
3.2.4 Asymptomatic PAD
Often, PAD lesions and/or reduced ABI are identified during screening or as incidental findings. This finding in and of itself is low risk but is a marker for elevated risk of other CV events such as CV death, MI, and stroke.[114] The focus for these patients should be on optimal medical therapy to reduce the risk of MACE; revascularization is generally not indicated unless progression is rapid.
Recommendation
45. We recommend not offering revascularization to patients with asymptomatic PAD (Strong Recommendation; Low-Quality Evidence).
Practical Tip
In patients with asymptomatic PAD, counsel and implement CV risk reduction therapies, in addition to providing education on signs and symptoms of progressive PAD.
3.3 Techniques for revascularization
PICO 3.3a: What are the available revascularization procedures in treating patients with PAD and their outcomes?
3.3.1 Endovascular revascularization
PICO 3.3b: What are the available endovascular revascularization procedures in treating patients with PAD and their outcomes?
3.3.1.1 Overview for endovascular revascularization
Endovascular therapies represent an area of innovation and advancement in the management of patients with PAD. These minimally invasive procedures use variations of balloon dilatation and stent technologies, via small pinhole incisions usually performed as day surgery cases. These procedures have become an especially attractive option for severely comorbid patients with chronic limb-threatening ischemia or claudication, in whom an open surgical revascularization procedure would not have been tolerated, is not feasible as a result of anatomy or conduit availability, or is undesirable because of concomitant morbidity and recovery time. Although less invasive, these endovascular therapies continue to face challenges with respect to limited durability compared with open surgical repair.[115],[116]
The objective of endovascular therapy is to target lesions that are hemodynamically significant, usually in the range of 75%-100% occlusion, with the goal of creating inline flow to the affected muscle group or ischemic tissue in the lower extremity. In addition to considering patient factors, anatomical characteristics of lesions considered favourable for endovascular therapy include short lesion length, stenosis (vs chronic total occlusions), proximal lesions (iliac vs femoropopliteal vs tibial), those with minimal calcification, and good distal runoff. Common femoral and profunda artery lesions are generally not considered for standard endovascular therapy.[117]–[120]
3.3.1.2 Endovascular procedures
See the 3.3.1.2 Endovascular Procedures section of the Supplementary Material for endovascular procedures.
3.3.1.3 Outcomes of endovascular revascularization
Endovascular revascularization outcomes are heterogeneous and, in many aspects, difficult to quantify. The immediate results of crossing a lesion and providing a treatment resulting in minimal residual stenosis are simple to measure as a procedural success. Other outcome measures such as patient-reported subjective outcomes, hemodynamic improvement, wound healing, and even vessel patency are limited by the inconsistency of definitions. The indication of the procedure, chronic limb-threatening ischemia vs claudication, can also affect outcomes because the severity and natural history of the disease is often worse in the former.
Outcomes are separated into anatomical regions: aortoiliac, infrainguinal, and infrapopliteal. The endovascular treatment of the aortoiliac segment has become the preferred option for the anatomically suitable because of the low incidence of morbidity and mortality compared with open surgical options.[117],[121],[122]
The common femoral artery area historically has been treated only with open surgery. This practice is currently challenged by studies indicating comparable results with endovascular treatment. The anatomic challenge of a flexible location beneath the inguinal ligament and the potential of covering the profunda femoral artery during possible stent placement must be weighed against the excellent results from a straightforward, open procedure. The consensus remains for operative intervention aside from high-risk anatomy or patient factors.[123]
The superficial femoral artery segment has multiple challenges, including lengthening or shortening, compression, and twisting of the vessel during regular everyday activity. These dynamic challenges have led to stent fractures, which might lead to premature occlusion and restenosis.[124] RCTs have suggested that balloon angioplasty is comparable with bare-metal stents (BMS) for short lesions but for longer disease segments greater than 5-6 cm, nitinol BMS show extended patency.[125],[126] BMS, although improved, suffer from recurrent in-stent restenosis in the medium- and long-term, leading to relatively low long-term patency.[127] Covered stents, which prevent tissue ingrowth aside from the proximal and distal edges, might provide some advantage. Nonrandomized industry-sponsored studies showed 1-year primary patency and secondary patency at 73% and 92% percent, respectively.[128] A smaller RCT did not show a benefit compared with BMS for long lesions.[129]
Frequent restenosis of treated lesions has led to randomized trials for drug-eluting technologies, including balloon and stent platforms. Recent data for DES have shown excellent results with 1 and 5 year patency rates at 86% and 66%, respectively. Symptomatic improvement occurred in 92% and 80% of patients during that same period. Registry and single-arm data have also shown dramatic benefits for DES.[71],[130]
Drug-eluting balloons have also shown benefits compared with standard balloon angioplasty in randomized trials.[131]–[133] A meta-analysis of drug-eluting balloons vs balloon angioplasty showed a significant reduction in restenosis rates in the former group. It also showed that higher drug concentrations of paclitaxel were associated with a superior reduction in restenosis than lower doses (3.0 vs 2.0 mg/mm2).[134]
A concern about higher mortality rates associated with drug-eluting technologies has led several governing bodies to add warnings to their use.[135],[136] However, more recent data have brought these safety concerns into question.[137],[138]
A recent network meta-analysis that compared 14 different treatment modalities (ie, atherectomy, brachytherapy, cryoplasty, cutting balloons, drug-coated balloons, bare nitinol stents, DES, covered stents, and combinations), showed DES and covered stents to be the best modalities at 12 months and 24 months, respectively, for restenosis and target lesion revascularization.[139]
Infrapopliteal endovascular treatment has been associated with a high incidence of restenosis of the treated vessel, where primary patency rates range from 22% to 92% at 1 year.[140],[141] Although rates of restenosis or occlusion are high, limb salvage can be obtained and maintained despite relatively poor vessel patency rates.
Recommendations
46. We recommend endovascular therapy in appropriately selected patients with claudication or chronic limb-threatening ischemia (Strong Recommendation; Low-Quality Evidence).
47. We recommend against performing endovascular therapy in the common femoral or profunda femoris arteries (Strong Recommendation; Low-Quality Evidence).
48. We recommend against performing endovascular therapy for lesions in asymptomatic patients or lesions that are not hemodynamically significant (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation places a high value on offering a revascularization option in appropriately selected patients that balances the inherent advantages of reduced morbidity, mortality, and faster recovery with the disadvantages of limited durability and increased potential for interventions.
Practical Tip
Patients with claudication that are selected for endovascular therapy ideally have short stenotic or occlusive lesions in the iliac or superficial femoral artery and reasonable expectation for functional capacity. Endovascular therapy is a reasonable minimally invasive option for limb salvage in patients with chronic limb-threatening ischemia and limited life expectancy, increased number of comorbidities, limited conduit options, or hostile tissue environments including active infection, scarring from multiple repeat surgeries, or radiation.
3.3.2 Open and hybrid surgical revascularization
PICO 3.3c: What are the available open surgical and hybrid (endovascular and open surgical) revascularization procedures in treating patients with PAD and their outcomes?
3.3.2.1 Open surgical revascularization
Despite the increasing use of endovascular interventions, open surgery remains an important therapeutic option in selected patients with PAD. Atherosclerotic disease of the lower extremities is usually described or divided into inflow (aortoiliac) and outflow (infrainguinal) disease.
Endarterectomy is a technique in which the plaque is directly removed from the artery. This is a local open surgical repair and can be used as the sole procedure to treat short segment stenoses or occlusions. The artery can be closed primarily after the endarterectomy but is usually closed with a vein or prosthetic patch angioplasty to increase the diameter of the artery. Endarterectomy can also be used to treat and improve the inflow or outflow vessel(s) in conjunction with surgical bypass procedures. The common femoral artery is the most common lower extremity artery treated with endarterectomy as a sole procedure or at the time of bypass.
Aortoiliac (inflow) disease can be successfully treated surgically with the use of larger-calibre prosthetic bypasses that are associated with good patency rates (aortofemoral 90% at 5 years) and superior to endovascular revascularization in a recent meta-analysis.[121],[142] Lower but acceptable 5-year graft patency are noted with less direct inflow from femoral-femoral (60%-80%) or axillo-femoral bypasses (80%).[143]–[146]
However, all bypasses are associated with a low but significant incidence of operative, wound, and graft complications, which are avoided using the endovascular route. Aortoiliac disease is therefore generally first treated using endovascular techniques (angioplasty with or without stent) but if this is not possible or has failed, then surgical bypass can be considered in selected patients with acceptable risk profiles.
Infrainguinal (outflow) disease can be treated with bypass generally originating from the common femoral artery as inflow and terminating at the above- or below-knee popliteal, or tibial or pedal vessels. Open bypass procedures might be considered for long-segment occlusions that cannot be treated with endovascular techniques or local repairs alone.
Bypass patency is generally highest with the use of autogenous (saphenous or other) vein grafts. These bypasses have patency rates that range from 60% to 80% at 5 years for popliteal vein bypasses.[147] Patency decreases significantly with more distal tibial or pedal artery bypasses, and therefore should only be performed for chronic limb-threatening ischemia. Patency rates are lower with the use of prosthetics at these levels and are avoided if possible.[147]
3.3.2.2 Hybrid procedures
“Hybrid” procedures involve the concurrent use of surgical and endovascular techniques for revascularization. They offer the advantages of less invasive procedures (potential for less local and systemic morbidity, and quicker recovery time) in circumstances in which endovascular treatment alone might be insufficient or is not anatomically feasible. In these procedures, an angioplasty with or without stent is performed proximal or distal to a surgical bypass or endarterectomy during the same sitting to optimize inflow or outflow. This offers a more complete revascularization while limiting surgical exposure and operative time. Recently, these hybrid procedures have been increasingly used as revascularization options for patients.[148] Choosing between exclusively surgical, endovascular, or hybrid revascularization will depend on patient anatomy and condition, and will take into consideration the feasibility, near-term risks, and long-term durability of the procedure(s).
Recommendations
Surgical revascularization for intermittent claudication
49. We recommend that surgical bypass to the popliteal artery (when indicated) should be performed with an autogenous vein in preference to prosthetic graft material for the treatment of intermittent claudication (Strong Recommendation; High-Quality Evidence).
50. We recommend against performing femoral-tibial artery bypasses for the treatment of intermittent claudication (Strong recommendation; Moderate-Quality Evidence).
Surgical revascularization for chronic limb-threatening ischemia
51. We recommend that surgical bypass to the popliteal or infrapopliteal arteries should be performed with an autogenous vein for chronic limb-threatening ischemia (Strong Recommendation; High-Quality Evidence).
52. We suggest that in patients with chronic limb-threatening ischemia for whom endovascular revascularization is not feasible and a suitable autogenous vein is not available, prosthetic material can be effective for bypass to the below-knee popliteal and tibial arteries as a last resort in cases of limb salvage (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation emphasizes the use of an autogenous vein as the best conduit for surgical bypass regardless of the indication. The choice of surgical conduit can significantly affect longer-term patency and limb salvage. For example, an autogenous saphenous vein has the best long-term patency rates in open revascularization procedures. The use of arm or composite vein grafts results in decreased but acceptable patency. The use of prosthetic bypasses below the knee generally results in poor long-term patency rates.
Recommendation
53. We suggest a staged (proximal revascularization of aortic inflow first) approach to surgical procedures is reasonable in patients with ischemic rest pain (Weak Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation emphasizes that the extent of revascularization will depend on the severity of presentation. Patients with rest pain, in contrast to patients with significant ulcers or gangrene, might not require all levels of disease to be revascularized to resolve their symptoms. If indicated, and technically or anatomically feasible, endovascular treatment of aorta-iliac disease before, or concurrent with, infrainguinal surgical procedures offers a less invasive approach that can improve inflow and can contribute to the success of revascularization.
3.4 Choosing between open surgical and endovascular procedures
PICO 3.4: How to choose between open surgical and endovascular procedures in patients with PAD who require revascularization procedures?
3.4.1 Evidence for open surgical vs endovascular revascularization
3.4.1.1 Claudication
There are no contemporary trials that have compared surgical with endovascular revascularization in patients with claudication related to aortoiliac and/or infrainguinal artery occlusive disease. Because of the previously discussed recommendation of offering the lowest possible risk intervention when moving forward with revascularization for debilitating claudication, it is unlikely that equipoise needed for a trial will ever exist.
3.4.1.2 Chronic limb-threatening ischemia
Only 1 RCT has been completed on this topic and open surgical bypass was compared with balloon angioplasty in patients with chronic limb-threatening ischemia due to infrainguinal arterial occlusive disease.[149] There were no differences in amputation-free survival at 1 or 5 years. However, post hoc analysis suggested greater amputation-free survival with bypass surgery starting 2 years after randomization.[108] This finding supports the superior durability of surgical bypass relative to balloon angioplasty in patients with chronic limb-threatening ischemia.
There are, however, no trials that have compared open surgical vs endovascular revascularization that include: (1) aortoiliac disease; (2) the full spectrum of open (eg, endar-terectomy) and hybrid revascularization (eg, iliac stent and femoral patch angioplasty); or (3) the full spectrum of contemporary endovascular revascularization approaches.
3.4.2 Decision determinants for open vs endovascular
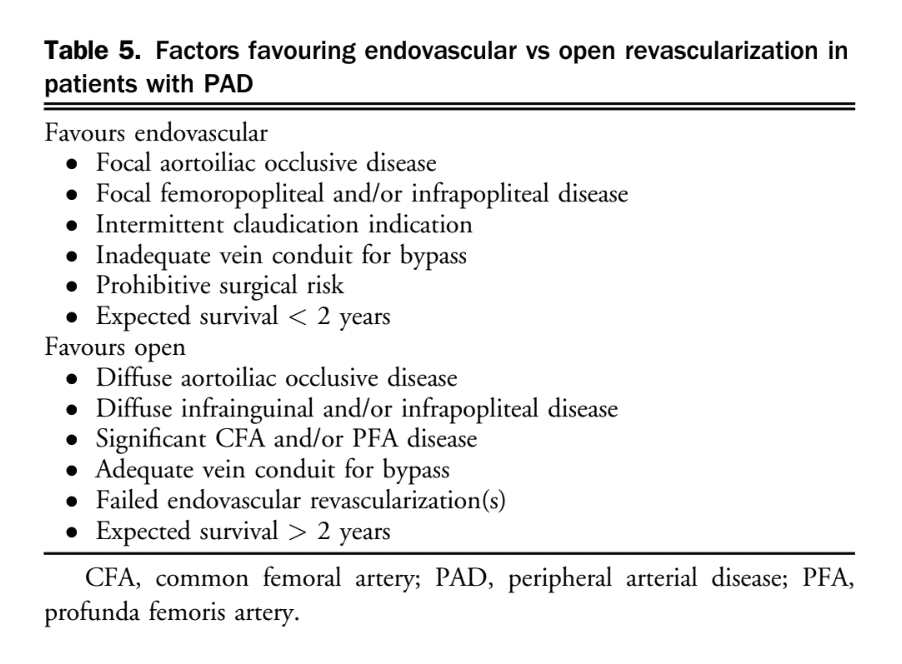
After appropriate imaging is obtained and a decision has been made for revascularization, careful consideration of anatomic, patient, and procedural factors is essential to select the optimal revascularization strategy (Table 5).
Recommendation
54. We recommend that when selecting endovascular vs open revascularization strategies for PAD, one must consider anatomic, patient, and procedural factors, in addition to operator expertise and resource availability (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation places a high value on individualizing revascularization strategies for PAD patients.
Practical Tips
Hybrid revascularization can be considered in those with common femoral or profunda femoral occlusive disease requiring endarterectomy, in addition to inflow and/or outflow disease amenable to endovascular therapy.
3.4.3 Upcoming trials
Two ongoing clinical trials of endovascular vs open revascularization for patients with PAD have the potential to change practice in the near future. The Best Endovascular vs Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST-CLI) and Bypass vs Angioplasty in Severe Ischemia of the Leg (BASIL)-2.[150],[151]
References
90. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol 2017;33:17-32.
91. Bertges DJ, Goodney PP, Zhao Y, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J Vasc Surg 2010;52:674-83. 683.e1-3.
92. Bertges DJ, Neal D, Schanzer A, et al. The Vascular Quality Initiative Cardiac Risk Index for prediction of myocardial infarction after vascular surgery. J Vasc Surg 2016;64. 1411-21.e4.
93. Scarborough JE, Bennett KM, Englum BR, Pappas TN, Lagoo-Deenadayalan SA. The impact of functional dependency on outcomes after complex general and vascular surgery. Ann Surg 2015;261:432-7.
94. Brahmbhatt R, Brewster LP, Shafii S, et al. Gender and frailty predict poor outcomes in infrainguinal vascular surgery. J Surg Res 2016;201:156-65.
95. Visser L, Banning LBD, el Moumni M, Zeebregts CJ, Pol RA. The effect of frailty on outcome after vascular surgery. Eur J Vasc Endovasc Surg 2019;58:762-9.
96. Houghton JSM, Nickinson ATO, Morton AJ, et al. Frailty factors and outcomes in vascular surgery patients: a systematic review and meta-analysis. Ann Surg 2020;272:266-76.
97. Eslami MH, Saadeddin Z, Rybin DV, Doros G, Siracuse JJ, Farber A. Association of frailty index with perioperative mortality and in-hospital morbidity after elective lower extremity bypass. J Vasc Surg 2019;69:863-874.e1.
98. Kertai MD, Boersma E, Klein J, van Urk H, Poldermans D. Optimizing the prediction of perioperative mortality in vascular surgery by using a customized probability model. Arch Intern Med 2005;165:898-904.
99. Teixeira IM, Teles AR, Castro JM, Azevedo LF, Mourão JB. Physiological and Operative Severity Score for the enumeration of Mortality and Morbidity (POSSUM) system for outcome prediction in elderly patients undergoing major vascular surgery. J Cardiothorac Vasc Anesth 2018;32:960-7.
100. Reis P, Lopes AI, Leite D, et al. Incidence, predictors and validation of risk scores to predict postoperative mortality after noncardiac vascular surgery, a prospective cohort study. Int J Surg 2020;73:89-93.
101. Rodseth RN, Biccard BM, le Manach Y, et al. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J Am Coll Cardiol 2014;63:170-80.
102. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FGR. Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg 2007;45(suppl S):S5-67.
103. Aquino R, Johnnides C, Makaroun M, et al. Natural history of claudication: long-term serial follow-up study of 1244 claudicants. J Vasc Surg 2001;34:962-70.
104. Jelnes R, Gaardsting O, Jensen KH, BÆkgaard N, Tønnesen KH, Schroeder T. Fate in intermittent claudication: outcome and risk factors. Br Med J (Clin Res Ed) 1986;293:1137-40.
105. Hussain MA, Al-Omran M, Mamdani M, et al. Efficacy of a guideline recommended risk-reduction program to improve cardiovascular and limb outcomes in patients with peripheral arterial disease. JAMA Surg 2016;151:742-50.
106. Kullo IJ, Rooke TW. Peripheral artery disease. N Engl J Med 2016;374:861-71.
107. Shishehbor MH, White CJ, Gray BH, et al. Critical limb ischemia: an expert statement. J Am Coll Cardiol 2016;68:2002-15.
108. Farber A, Eberhardt RT. The current state of critical limb ischemia: a systematic review. JAMA Surg 2016;151:1070-7.
109. Hussain MA, Al-Omran M, Creager MA, Anand SS, Verma S, Bhatt DL. Antithrombotic therapy for peripheral artery disease: recent advances. J Am Coll Cardiol 2018;71:2450-67.
110. Olin JW, White CJ, Armstrong EJ, Kadian-Dodov D, Hiatt WR. Peripheral artery disease: evolving role of exercise, medical therapy, and endovascular options. J Am Coll Cardiol 2016;67:1338-57.
111. Abu Dabrh AM, Steffen MW, Undavalli C, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg 2015;62. 1642-51.e3.
112. Forsythe RO, Apelqvist J, Boyko EJ, et al. Effectiveness of revascularisation of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review. Diabetes Metab Res Rev 2020;36(suppl 1):e3279.
113. Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med 2012;366:2198-206.
114. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116:1509-26.
115. Antoniou GA, Chalmers N, Georgiadis GS, et al. A meta-analysis of endovascular versus surgical reconstruction of femoropopliteal arterial disease. J Vasc Surg 2013;57:242-53.
116. Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010;51(5 suppl):5S-17S.
117. Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. J Vasc Surg 2019;69:3S-125S.e40.
118. Löfberg AM, Karacagil S, Ljungman C, et al. Percutaneous transluminal angioplasty of the femoropopliteal arteries in limbs with chronic critical lower limb ischemia. J Vasc Surg 2001;34:114-21.
119. Capek P, McLean GK, Berkowitz HD. Femoropopliteal angioplasty. Factors influencing long-term success. Circulation 1991;83(2 supply):I70-80.
120. Clark TWI, Groffsky JL, Soulen MC. Predictors of long-term patency after femoropopliteal angioplasty: results from the STAR registry. J Vasc Interv Radiol 2001;12:923-33.
121. de Vries SO, Hunink MGM. Results of aortic bifurcation grafts for aortoiliac occlusive disease: a meta-analysis. J Vasc Surg 1997;26:558-69.
122. Jongkind V, Akkersdijk GJM, Yeung KK, Wisselink W. A systematic review of endovascular treatment of extensive aortoiliac occlusive disease. J Vasc Surg 2010;52:1376-83.
123. Boufi M, Ejargue M, Gaye M, Boyer L, Alimi Y, Loundou AD. Systematic review and meta-analysis of endovascular versus open repair for common femoral artery atherosclerosis treatment. J Vasc Surg 2021;73:1445-55.
124. Scheinert D, Scheinert S, Sax J, et al. Prevalence and clinical impact of stent fractures after femoropopliteal stenting. J Am Coll Cardiol 2005;45:312-5.
125. Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006;354:1879-88.
126. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation versus balloon angioplasty for lesions in the superficial femoral artery and proximal popliteal artery: twelve-month results from the RESILIENT randomized trial. Circ Cardiovasc Interv 2010;3:267-76.
127. Abdoli S, Katz S, Ochoa C. Long-term patency and clinical outcomes of nitinol stenting for femoropopliteal atherosclerotic disease. Ann Vasc Surg 2020;66:566-72.
128. Saxon RR, Chervu A, Jones PA, et al. Heparin-bonded, expanded polytetrafluoroethylene-lined stent graft in the treatment of femoropopliteal artery disease: 1-year results of the VIPER (Viabahn Endoprosthesis with Heparin Bioactive Surface in the Treatment of Superficial Femoral Artery Obstructive Disease) trial. J Vasc Interv Radiol 2013;24:165-73 [quiz: 174].
129. Geraghty PJ, Mewissen MW, Jaff MR, Ansel GM. Three-year results of the VIBRANT trial of VIABAHN endoprosthesis versus bare nitinol stent implantation for complex superficial femoral artery occlusive disease. J Vasc Surg 2013;58:386-395.e4.
130. Stavroulakis K, Torsello G, Bosiers M, Argyriou A, Tsilimparis N, Bisdas T. 2-Year outcomes of the Eluvia drug-eluting stent for the treatment of complex femoropopliteal lesions. JACC Cardiovasc Interv 2021;14:692-701.
131. Schneider PA, Laird JR, Tepe G, et al. Treatment effect of drug-coated balloons is durable to 3 years in the femoropopliteal arteries: long-term results of the IN.PACT SFA randomized trial. Circ Cardiovasc Interv 2018;11:e005891.
132. Rosenfield K, Jaff MR, White CJ, et al. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med 2015;373:145-53.
133. Tepe G, Zeller T, Albrecht T, et al. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med 2008;358:689-99.
134. Caradu C, Lakhlifi E, Colacchio EC, et al. Systematic review and updated meta-analysis of the use of drug-coated balloon angioplasty versus plain old balloon angioplasty for femoropopliteal arterial disease. J Vasc Surg 2019;70:981-995.e10.
135. Dan K, Shlofmitz E, Khalid N, et al. Paclitaxel-related balloons and stents for the treatment of peripheral artery disease: insights from the Food and Drug Administration 2019 Circulatory System Devices Panel Meeting on late mortality: paclitaxel devices in PAD treatment. Am Heart J 2020;222:112-20.
136. Katsanos K, Spiliopoulos S, Kitrou P, Krokidis M, Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc 2018;7:e011245.
137. Choi H, Lee H, Lee SS, Ahn J, Joh JH, Lee MY. Association of mortality with drug-coated devices in femoropopliteal artery based on the nationwide data. Ann Surg Treat Res 2021;101:20-7.
138. Schneider PA, Laird JR, Doros G, et al. Mortality not correlated with paclitaxel exposure: an independent patient-level meta-analysis of a drug-coated balloon. J Am Coll Cardiol 2019;73:2550-63.
139. Zhou Y, Zhang Z, Lin S, et al. Comparative effectiveness of endovascular treatment modalities for de novo femoropopliteal lesions: a network meta-analysis of randomized controlled trials. J Endovasc Ther 2020;27:42-59.
140. Lipsitz EC, Veith FJ, Ohki T. The value of subintimal angioplasty in the management of critical lower extremity ischemia: failure is not always associated with a rethreatened limb. J Cardiovasc Surg (Torino) 2004;45:231-7.
141. Schmidt A, Ulrich M, Winkler B, et al. Angiographic patency and clinical outcome after balloon-angioplasty for extensive infrapopliteal arterial disease. Catheter Cardiovasc Interv 2010;76:1047-54.
142. Premaratne S, Newman J, Hobbs S, Garnham A, Wall M. Meta-analysis of direct surgical versus endovascular revascularization for aortoiliac occlusive disease. J Vasc Surg 2020;72:726-37.
143. Perler BA, Burdick JF, Williams GM. Femoro-femoral or ilio-femoral bypass for unilateral inflow reconstruction? Am J Surg 1991;161:426-30.
144. Blaisdell FW. Development of femoro-femoral and axillo-femoral bypass procedures. J Vasc Surg 2011;53:540-4.
145. Samson RH, Showalter DP, Lepore MR, Nair DG, Dorsay DA, Morales RE. Improved patency after axillofemoral bypass for aortoiliac occlusive disease. J Vasc Surg 2018;68:1430-7.
146. Nguyen KP, Perrone KH, Rahman A, et al. The role of axillofemoral bypass in current vascular surgery practice. Am J Surg 2016;211:968-71.
147. Almasri J, Adusumalli J, Asi N, et al. A systematic review and meta-analysis of revascularization outcomes of infrainguinal chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;58:S110-9.
148. Fereydooni A, Zhou B, Xu Y, Deng Y, Dardik A, Ochoa Chaar CI. Rapid increase in hybrid surgery for the treatment of peripheral artery disease in the Vascular Quality Initiative database. J Vasc Surg 2020;72:977-986.e1.
149. Bradbury AW, Adam DJ, Beard JD, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005;366:1925-34.
150. Menard MT, Farber A, Assmann SF, et al. Design and rationale of the Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST-CLI) Trial. J Am Heart Assoc 2016;5:e003219.
151. Popplewell MA, Davies H, Jarrett H, et al. Bypass versus Angio plasty in Severe Ischaemia of the Leg – 2 (BASIL-2) trial: study protocol for a randomised controlled trial. Trials 2016;17:11.
