1. Diagnosis and Screening
1.1. Diagnosis of PAD
PICO 1.1a: What are the most accurate signs, symptoms, and tests for detecting asymptomatic PAD in adults at risk of PAD?
PICO 1.1b: What are the most accurate signs, symptoms, and tests (Ankle-Brachial Index [ABI], Toe-Brachial Index [TBI], transcutanous oxygen pressure [TcP02], plethysmographic wave-forms, magnetic resonance angiography [MRA], computed tomography angiography [CTA], etc) for detecting PAD in adult men and women who present with lower limb symptoms?
PICO 1.1c: What are the most accurate signs, symptoms, and tests (ABI, TBI, TcPO2, plethysmographic waveforms, MRA, CTA, etc) for detecting PAD in adult men and women with diabetes or chronic kidney disease who present with lower limb symptoms?
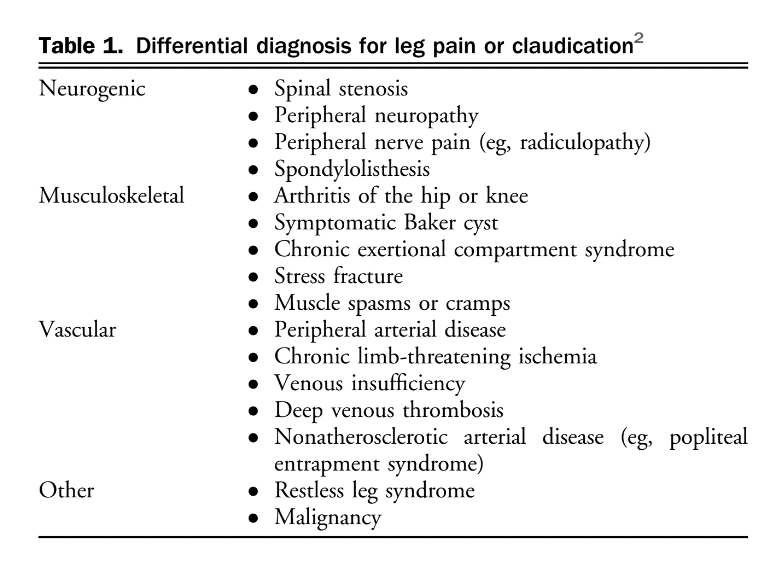
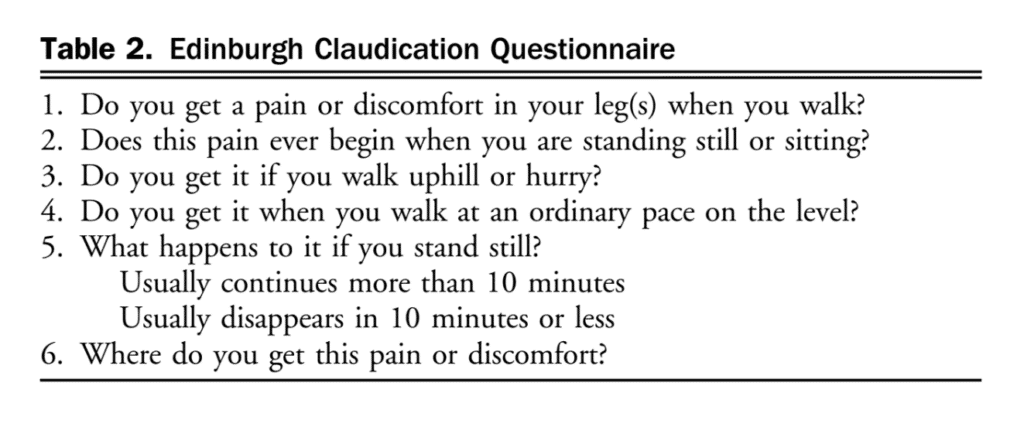
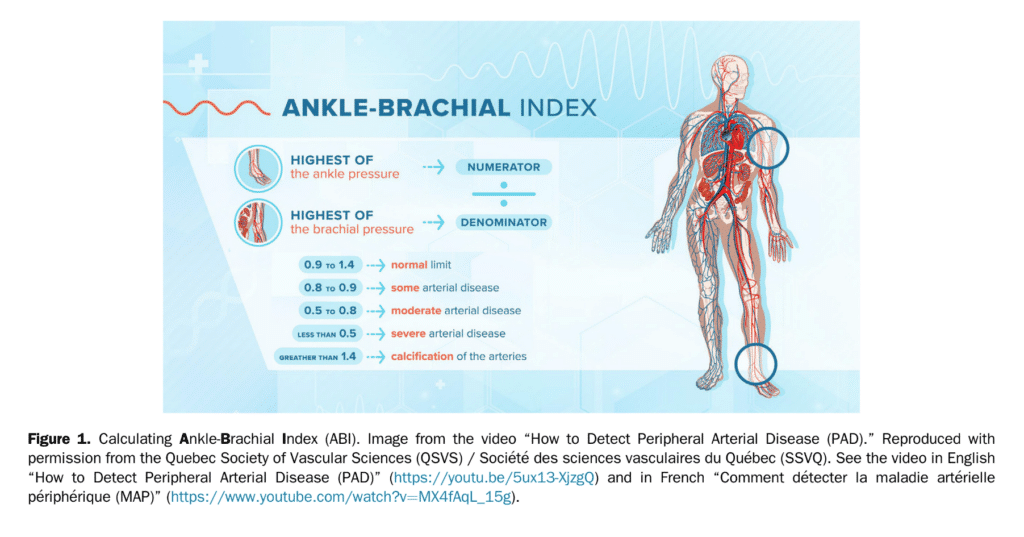
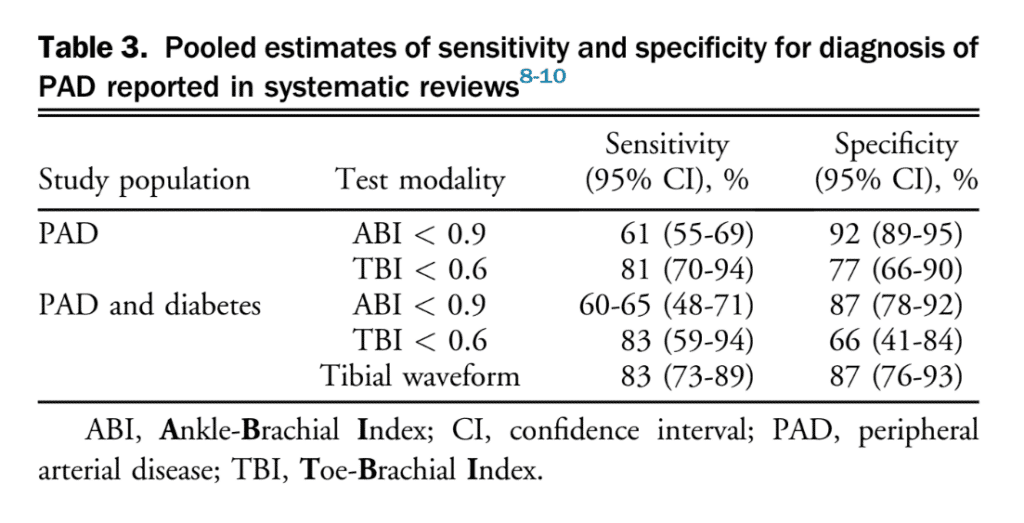
Lower extremity PAD is prevalent among people aged 50 years and older. Among those with lower limb symptoms suggestive of claudication, it is important for clinicians to distinguish PAD from other causes of leg pain (Table 1), starting with a thorough clinical history and physical examination focused on relevant signs and symptoms. A validated diagnostic questionnaire for PAD used in epidemiological studies is the Edinburgh Claudication Questionnaire (Table 2), which has a 91.3% (95% confidence interval [CI], 88.1%-94.5%) sensitivity and 99.3% (95% CI, 98.9%-100%) specificity.[3] Only 5%-10% of patients with PAD present with classical symptoms of intermittent claudication.[4] Other patients pre-sent with nonspecific back, buttocks, or leg discomfort, whereas some are asymptomatic. Classic features of claudication include: (1) muscle pain, typically involving calf muscles or the muscle group distal to an arterial tenosis or occlusion and often described as cramping in nature; (2) pain that develops only when the muscle is exercised; and (3) pain that resolves usually within 10 minutes of discontinuation of exercise or resting. Typically, patients with vasculogenic claudication experience cramping muscle pain after walking a similar distance. Intermittent claudication differs from chronic limb-threatening ischemia, which includes ischemic rest pain, gangrene, or ulceration on the lower extremity. After a thorough clinical history, clinicians should conduct a focused peripheral vascular examination. Confirmation of the diagnosis of PAD then requires specific tests, considering the broad differential for leg pain (see section 1.1 of the Supplementary Material for more information). There are a number of confirmatory diagnostic tests that help establish the diagnosis of PAD. Some of these tests are more invasive and others are specialized and/or centre-specific. The most widely used test is the ABI. The ABI is an inexpensive, noninvasive test that involves measuring the systolic BP at the arm (or over the brachial artery) and ankle (or over the dorsal pedis or posterior tibial artery) while the patient is supine, using a continuous-wave Doppler device (Fig. 1). The higher value of systolic pressure at the ankle is divided by the higher of the arm pressures (right or left) to obtain the ABI.[5],[6] The most widely used and accepted ABI calculation is shown in Figure 1. An ABI < 0.9 suggests PAD.[5] The incidence of PAD varies according to the prevalence of risk factors for the disease such as smoking, hypertension, hypercholesterolemia, and diabetes mellitus.[4] Medial arterial calcinosis, which is more prevalent in patients with diabetes, chronic kidney disease, and advanced age, results in poorly compressible arteries.[7] This might falsely normalize or artificially elevate ABI to a value exceeding 1.4, rendering this test less reliable. Many studies have investigated the accuracy of ABI, oscillometric ABI, TBI, near-infrared technology, pulse oximetry, pulse wave velocity, transcutaneous oxygenation, computed tomography, magnetic resonance imaging, and conventional angiography for diagnosing PAD. The TBI can be calculated with arm and great toe arterial BP measurements. The great toe systolic pressures are divided by the highest arm pressure to establish a TBI measurement for each leg. ABI and TBI are the most studied, but there is a paucity of data for most other tests for the diagnosis of PAD. From these accuracy studies, test characteristics have a very wide range depending on symptoms and risk factors of the population (eg, sensitivity of 45%-100% and specificity of 16%-100% for TBI).[7] There is also a lack of consistency with respect to how to perform an ABI or TBI. With TBI, the diagnostic cutoff for PAD is variable among studies, although < 0.60 is commonly used. Data suggest that ABI is a reliable way to diagnose PAD. Table 3 shows a comparison of general sensitivity and specificity ranges for these test modalities. An ABI might be insufficient to be used alone for the detection of PAD in people with diabetes and chronic kidney disease because of the higher probability of medial calcification. The literature is limited on diagnosis in patients with chronic kidney disease. Other tests such as the TBI, tibial waveform, and/or transcutaneous oxygenation should be considered for diagnosing PAD in the case that ABI > 1.4, suggestive of calcified arteries.
1.1.1 Sex/gender differences
Although population-based studies suggest higher prevalence of asymptomatic disease and more complex or multilevel and severe disease at the time of diagnosis for women, there is a paucity of studies that specifically examined sex differences in diagnosis of PAD.[11]–[13] However, inclusion of the ABI in CV risk stratification resulted in reclassification of the risk category and modification of treatment in approximately 19% of men and 36% of women.[14] The effect of sex and gender on PAD presentation and diagnostic tools such as ABI and TBI warrant further study.
Recommendation
- We suggest using the ABI for asymptomatic adults, older than age 50 years, who have risk factors for PAD (such as smoking or diabetes), to screen for PAD (Weak Recommendation; Low-Quality Evidence).
Recommendation
- We recommend using an ABI and/or a TBI study to confirm the diagnosis of PAD in patients with symptoms of PAD (Strong Recommendation; Moderate-Quality Evidence).
Recommendation
- We suggest using a TBI with tibial waveforms and/or transcutaneous oxygen pressure as adjuncts for patients with symptoms of PAD with calcified arteries to confirm the diagnosis of PAD (Weak Recommendation; Low-Quality Evidence).
Values and Preferences
To improve patient’s quality and quantity of life in the long-term, it is important to diagnose PAD in at-risk asymptomatic, and symptomatic patients to initiate appropriate best, evidence-based, medical therapy.
Practical Tip
The ABI used in the diagnosis of PAD can be easily classified and measured. See Figure 1 and video links provided in the legend.
1.2 Asymptomatic PAD Screening
PICO 1.2a: In patients without claudication symptoms, but at high risk for clinically apparent atherosclerotic disease, would routine systematic screening for PAD improve lower limb ischemia outcomes?
PICO 1.2b: In patients without claudication symptoms, but who have documented atherosclerosis in another vascular bed, would routine systematic screening for PAD improve global CV outcomes?
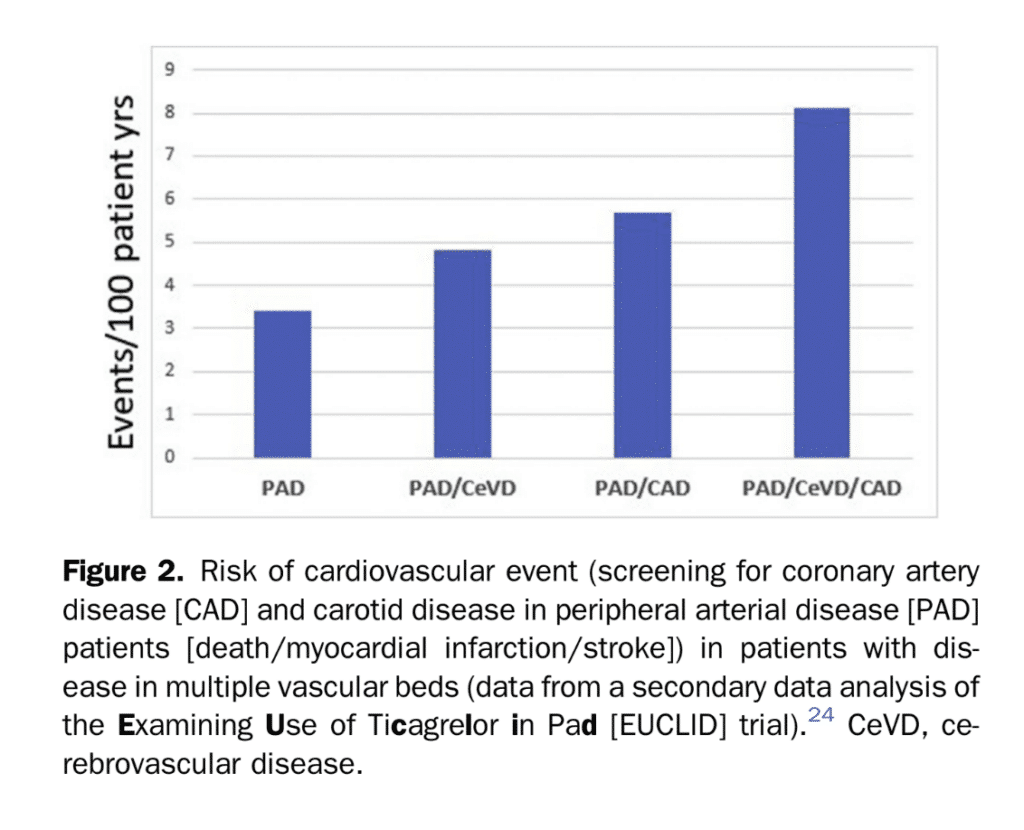
The ideal characteristics for a successful population-based screening strategy would include use of a test with high sensitivity that leads to efficacious therapy, if used early in the disease state. Although ABI measurement has very high specificity for detecting PAD, there are no current recommendations for intervention on asymptomatic disease because there has been no demonstration of benefit.[15],[16] The Society for Vascular Surgery and the Canadian Society for Vascular Surgery recommend against invasive treatments for asymptomatic PAD (percutaneous or surgery), regardless of ABI or other test results that indicate PAD.[17],[18] A recent systematic review including studies of asymptomatic patients demonstrated no benefit in major adverse CV events (MACE) or quality of life measures with intervention.[18] A meta analysis commissioned by the Society for Vascular Surgery did not yield evidence of either cost effectiveness for ABI screening of asymptomatic patients, or any clear reduction in morbidity or mortality.[14] Individuals with multiple vascular territory involvement or polyvascular disease carry worse prognosis when identified (Fig. 2).[19]–[22] To date, there has been no RCT to suggest that the strategy of systematic screening in this setting is effective in lowering rates of CV events, or MALE (evolution to limb-threatening ischemia or limb loss).[18] This is likely because risk factor management and pharmacotherapy are not vastly different when single or multiple vascular beds are involved. Similarly, current guidelines for dyslipidemia, hypertension, or antithrombotic agents have not delineated different treatment streams for patients with vascular disease in multiple vascular territories. However, novel agents, such as PCSK-9 inhibitors and low-dose direct oral anticoagulation were not used in previous studies. It is unknown if diagnosing those at higher risk for events would change management strategies and outcomes. As we move to precision medicine, the use of the ABI might further refine global CV risk assessment. An abnormal ABI is prognostic, regardless of symptoms. The utility of such a strategy might be dependent on the stage of a patient’s disease. ABIs and CTA delineate flow-limiting lesions. Abnormal results would be a sign of late disease. They are less helpful when trying to detect preclinical or early stage atherosclerosis in a relatively low risk primary prevention population. However, further refinement of risk estimates in secondary prevention populations could assist in determining the risk-benefit of certain pharmacotherapies and optimize prescription of high-cost treatments. A higher absolute CV risk in a patient with polyvascular disease might justify prescription of a more potent antithrombotic that has a greater bleeding risk,[19],[21],[22] or expensive agents such as PCSK-9 inhibitors. Thus far, we do not have a global vascular risk-estimate tool that has led to ischemic event reduction. A recent cost effectiveness analysis tested the use of ABI screening to detect asymptomatic PAD in patients with recently symptomatic CAD. The analysis showed that patients with asymptomatic PAD had minimal quality of life years gained, with significantly high incremental cost, much above usual willingness to pay thresholds.[23]
Recommendations
- We recommend against implementing a broad, population-based screening strategy for PAD, in patients without signs or symptoms of claudication (Strong Recommendation; High-Quality Evidence).
Values and Preferences
In contrast to general population screening, individual patients with high-risk features such as smoking and diabetes, might benefit from identification of PAD and more aggressive medical management. However, patients do not benefit from revascularization with asymptomatic PAD. Patients’ interest in their future vascular risk should be considered when determining if ABI testing will be undertaken.
Practical Tip
Patients should be assessed and managed for vascular risk factors. Focus should be on the management of CV risk factors rather than the pursuit of revascularization. Currently available tests for PAD do not assess for preclinical disease. Other modalities to assess preclinical disease in coronary and carotid arteries have greater evidence to assist in global CV risk assessment in a primary prevention setting.
Recommendations
- We suggest against routine PAD testing for inferring global CV risk, in patients without symptoms of PAD, who have clinically symptomatic atherosclerosis in another vascular territory (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
For patients with polyvascular disease, strong evidence for routine screening of other vascular territories is lacking. There is lack of consensus on how this screening would differentiate treatment. We could not recommend this practice. However, the committee recognizes that in select patients, identifying a second vascular bed might change management and assist in overcoming financial barriers for certain pharmaceuticals.
Practical Tip
Global CV risk reduction should focus on risk factor management as suggested in respective risk factor guidelines.
1.3 Screening for CAD and carotid disease in PAD patients
PICO 1.3: Does screening patients with PAD for CAD or cerebrovascular disease reduce myocardial infarction (MI), stroke, or CV death?
Recommendation
- We suggest against routine screening for asymptomatic carotid artery stenosis or asymptomatic CAD, among patients with documented PAD (Weak Recommendation; Low-Quality Evidence).
Values and Preferences
Most patients with PAD require aggressive CV risk factor management to improve long-term outcomes. This is on the basis of the strong epidemiological evidence linking PAD with incident MI and stroke.
Practical Tip
Patients with PAD have a high incidence of concomitant disease in other vascular beds and should be questioned about symptoms attributable to those vascular beds. Patients with polyvascular disease are a particularly high-risk group for future CV events and warrant aggressive management of standard atherosclerotic risk factors, including smoking, hypertension, dyslipidemia, and diabetes.
References
2. Hennion DR, Siano KA. Diagnosis and treatment of peripheral arterial disease. Am Fam Physician 2013;88:306-10.
3. Lend GC, Fowkes FGR. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose questionnaire for use in epidemiological surveys. J Clin Epidemiol 1992;45:1101-9.
4. Bauersachs R, Debus S, Nehler M, et al. A targeted literature review of the disease burden in patients with symptomatic peripheral artery disease. Angiology 2020;71:303-14.
5. Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the Ankle-Brachial Index: a scientific statement from the American Heart Association. Circulation 2012;126:2890-909.
6. Khan NA, Rahim SA, Anand SS, Simel DL, Panju A. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA 2006;295:536-46.
7. Tehan PE, Santos D, Chuter VH. A systematic review of the sensitivity and specificity of the toe-brachial index for detecting peripheral artery disease. Vasc Med 2016;21:382-9.
8. Herraiz-Adillo Á, Cavero-Redondo I, Álvarez-Bueno C, Pozuelo-Carrascosa DP, Solera-Martínez M. The accuracy of toe brachial index and ankle brachial index in the diagnosis of lower limb peripheral arterial disease: a systematic review and meta-analysis. Atherosclerosis 2020;315: 81-92.
9. Chuter VH, Searle A, Barwick A, et al. Estimating the diagnostic accuracy of the ankleebrachial pressure index for detecting peripheral arterial disease in people with diabetes: a systematic review and meta-analysis. Diabet Med 2021;38:e14379.
10. Normahani P, Mustafa C, Shalhoub J, et al. A systematic review and meta-analysis of the diagnostic accuracy of point-of-care tests used to establish the presence of peripheral arterial disease in people with diabetes. J Vasc Surg 2021;73:1811-20.
11. Higgins P, Higgins A. Epidemiology of peripheral arterial disease in women. J Epidemiol 2003;13:1-14.
12. Ortmann J, Nüesch E, Traupe T, Diehm N, Baumgartner I. Gender is an independent risk factor for distribution pattern and lesion morphology in chronic critical limb ischemia. J Vasc Surg 2012;55:98-104.
13. Sigvant B, Wiberg-Hedman K, Bergqvist D, et al. A population-based study of peripheral arterial disease prevalence with special focus on critical limb ischemia and sex differences. J Vasc Surg 2007;45:1185-91.
14. Alahdab F, Wang AT, Elraiyah TA, et al. A systematic review for the screening for peripheral arterial disease in asymptomatic patients. J Vasc Surg 2015;61(3suppl):42S-53S.
15. Guirguis-Blake JM, Evans CV, Redmond N, Lin JS. Screening for peripheral artery disease using the Ankle-Brachial Index updated evidence report and systematic review for the US preventive services task force. JAMA 2018;320:184-96.
16. Conte MS, Pomposelli FB, Clair DG, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg 2015;61(3 suppl):2S-41S.
17. Canadian Society for Vascular Surgery. Choosing Wisely Canada Recommendations: Vascular Surgery. Available at: https://choosingwiselycanada.org/vascular-surgery. Accessed February 20, 2022.
18. Collet JP, Cayla G, Ennezat PV, et al. Systematic detection of polyvascular disease combined with aggressive secondary prevention in patients presenting with severe coronary artery disease: the randomized AMERICA study. Int J Cardiol 2018;254:36-42.
19. Anand SS, Eikelboom JW, Dyal L, et al. Rivaroxaban plus aspirin versus aspirin in relation to vascular risk in the COMPASS Trial. J Am Coll Cardiol 2019;73:3271-80.
20. Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER trial (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk). Circulation 2018;137:338-50.
21. Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol 2016;67:2719-28.
22. Darmon A, Sorbets E, Ducrocq G, et al. Association of multiple enrichment criteria with ischemic and bleeding risks among COMPASS-eligible patients. J Am Coll Cardiol 2019;73:3281-91.
23. Minami H, Itoga NK, George EL, Garcia-Toca M. Cost-effectiveness analysis of Ankle-Brachial Index screening in patients with coronary artery disease to optimize medical management. J Vasc Surg 2021;74. 2030-9.e2.
24. Gutierrez JA, Mulder H, Jones WS, et al. Polyvascular disease and risk of major adverse cardiovascular events in peripheral artery disease: a secondary analysis of the EUCLID trial. JAMA Netw Open 2018;1:e185239.
