5. New Evidence for SGLT2 Inhibitors and HF
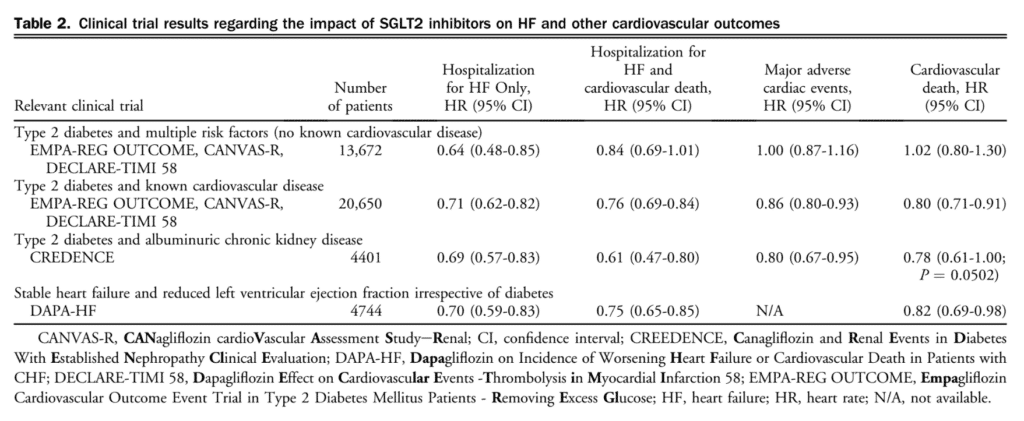
SGLT2 inhibitors lead to a reduction in plasma glucose by inhibiting renal tubular glucose reabsorption, with resultant glucosuria. These glycemia-related changes are also associated with natriuresis, an osmotic diuresis, modest weight loss, an increase in hematocrit, and a reduction in blood pressure. All of these effects represent potentially favourable changes that might lead to a reduction in incident HF in patients with type 2 diabetes, as initially shown in the landmark Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients – Removing Excess Glucose (EMPA-REG OUTCOME) trial.[41] In 2017, the CCS HF guidelines[15] recommended the use of SGLT2 inhibitors for prevention of HF events in patients with type 2 diabetes and known history of cardiovascular disease. In view of the rapidly evolving research on this topic, we have updated our recommendations. The SGLT2 inhibitor empagliflozin was the first glucose-lowering drug to show an improvement in cardiovascular outcomes in a large randomized controlled clinical trial.[42] The EMPA-REG OUTCOME trial randomized 7020 patients with type 2 diabetes and established cardiovascular disease (with estimated glomerular filtration rate [eGFR] 30 mL/min/1.73 m2) to receive either empagliflozin 10 mg or 25 mg or placebo. The study showed a statistically significant reduction in the composite outcome of cardiovascular death or HHF (HR, 0.83; 95% CI, 0.73-0.95; P 1⁄4 0.005) in the empagliflozin group vs placebo. A prespecified outcome, adjudicated HHF, was also significantly reduced with empagliflozin, with an HR of 0.73 (95% CI, 0.61-0.88).[42] The benefit of empagliflozin was observed in patients with and without an investigator-reported history of HF, and unrelated to baseline level of renal function (eGFR) or conventional risk factors, such as A1c, blood pressure, or lipids.[41] The Canagliflozin Cardiovascular Assessment Study-Renal (CANVAS-R) Program was the second major study of an SGLT2 inhibitor to show cardiovascular benefit in patients with type 2 diabetes.[43] In total, 10,142 patients with type 2 diabetes (eGFR > 30 mL/min/1.72 m2) with established cardiovascular disease or aged > 50 years with 2 risk factors for cardiovascular disease were randomized to receive canagliflozin (either 100 or 300 mg/d), or placebo.[44] The Canagliflozin Cardiovascular Assessment Study (CANVAS) Program also showed a 33% reduction in HF hospitalization with canagliflozin therapy (HR, 0.67; 95% CI, 0.52-0.87). A subsequent analysis[45] showed that this benefit occurred in patients with and without a preexisting atherosclerotic cardiovascular disease. In the Dapagliflozin Effect on Cardiovascular Events (DECLARE)-Thrombolysis in Myocardial Infarction (TIMI) 58 study,[46] dapagliflozin vs placebo was evaluated in 17,160 patients, 10,186 of whom did not have documented existing atherosclerotic cardiovascular disease.[47] Furthermore, unlike the previous studies, patients with a creatinine clearance of 60 mL or more per minute were enrolled. The primary safety outcome was a composite of major adverse cardiovascular events (MACE), defined as cardiovascular death, myocardial infarction, or ischemic stroke. The primary efficacy outcomes were MACE and a composite of cardiovascular death or HHF. The rates of MACE were not statistically different (HR, 0.93; 95% CI, 0.84-1.03; P 1⁄4 0.17), however, the composite of cardiovascular death or HHF was reduced (HR, 0.83; 95% CI, 0.73-0.95; P 1⁄4 0.005). The latter finding was driven primarily by a reduction in HHF (HR, 0.73; 95% CI, 0.61-0.88) and similar relative benefits were observed in patients with or without preexisting atherosclerotic cardiovascular disease or HF. In a secondary analysis of the DECLARE study, 671 patients were noted to have an LVEF < 45% (documented in patients with available echocardiograms) whereas 1316 had a history of HF without a reduced EF (808 with a documented EF 45% and 508 without a documented EF).[48] In this analysis, subjects with LVEF < 45% treated with dapagliflozin derived a larger decrement of cardiovascular death or HF hospitalization (HR, 0.62; 95% CI, 0.45-0.86) compared with those without known lower LVEF (HR, 0.88; 95% CI, 0.76-1.02; P interaction 1⁄4 0.046), the difference driven by a reduction in cardiovascular mortality (HR, 0.55; 95% CI, 0.34-0.90). In addition, only those with lower EF treated with dapagliflozin experienced a reduction in all-cause mortality (HR, 0.59; 95% CI, 0.40-0.88; P 1⁄4 0.01). Because these analyses were not prespecified, the findings are hypothesis-generating and require confirmation in prospective studies of well characterized patients with established HF. Preexisting renal disease is a known risk factor for cardiovascular death and HF hospitalization. Recently the results of Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation (CREDENCE),[49] the first renal-dedicated SGLT2 inhibitor trial has been reported. In this trial, 4401 patients with type 2 diabetes and chronic albuminuric renal disease (eGFR 30-90 mL/min/1.73 m2) were randomized to receive placebo or canagliflozin 100 mg/d. Importantly, all patients were required to be receiving background stable doses of ACE inhibitor or ARB for at least a 4-week period. The trial was stopped early for efficacy at a median follow-up of 2.6 years and showed a 30% lower rate in the primary outcome of renal progression or cardiovascular death (HR, 0.70; 95% CI, 0.59-0.82; P 1⁄4 0.00001). In addition, lower rates of HHF (HR, 0.61; 95% CI, 0.47-0.80; P < 0.001) were observed. There was no significant between group difference in the risk of cardiovascular death (HR, 0.78; 95% CI, 0.61-1.00; P 1⁄4 0.05) or in the risk of death from any cause (HR, 0.83; 95% CI, 0.68-1.02). These findings confirm the renal benefits observed in subanalyses from the earlier prevention trials and extend the HF benefits of SGLT2 inhibitors to patients with albuminuric chronic kidney disease. The TIMI Study Group recently published a systematic review and meta-analysis of 3 large trials of SGLT2 inhibitors: EMPA-REG OUTCOME, the CANVAS Program and DECLARE-TIMI 58.[50] The meta-analysis included 34,322 patients, of whom, 60.2% had established cardiovascular disease. Overall, patients treated with SGLT2 inhibitors experienced a lower rate of nonfatal myocardial infarction, stroke, or cardiovascular death (HR, 0.89; 95% CI, 0.83-0.96; P 1⁄4 0.0014), however, this benefit was limited to patients with established cardiovascular disease. The composite rate of cardiovascular death or HHF was reduced by 23% (HR, 0.77; 95% CI, 0.71-0.84; P < 0.001) and the rate of HHF alone was reduced by 31% (HR, 0.69; 95% CI, 0.61-0.7, P < 0.001) in patients who received SGLT2 inhibitors.[50] The benefits of SGLT2 inhibitors on preventing hospitalizations for HF were observed to a greater degree in those with mild to moderate renal dysfunction and with a history of HF, although only 10%-15% of patients with diabetes included in this meta-analysis had a history of HF. Taken together, the available data clearly show the efficacy of SGLT2 inhibition to reduce incident HF in a broad group of patients with type 2 diabetes.
Established HF due to reduced LVEF
The landmark study to evaluate the effect of Dapagliflozin on Incidence of Worsening Heart Failure or Cardiovascular Death in Patients with CHF (DAPA-HF) has recently been pubslished.[51] In this large, international, multicentre, double-blind, parallel, randomized, placebo-controlled trial 4744 patients were enrolled with stable class II-IV HFrEF and receiving optimal medical therapy and serum N-terminal pro-B-type natriuretic peptide > 600 pg/mL (> 400 if hospitalized within the past year, > 900 if atrial fibrillation or flutter present) and eGFR > 30 mL/min/1.73 m2 to either placebo or dapagliflozin 10 mg daily. Baseline characteristics and medical therapy of study participants in this study were well balanced between the two groups and were similar to those of other recently reported large randomized trials of HFrEF. Importantly, patients with (45%) and without (44%) diabetes were included in this event-driven study. Over a median 18-month follow-up, treatment with dapagliflozin reduced the primary end point, the composite of time to first worsening of HF (hospitalization or urgent visit requiring intravenous therapy for HF) or death due to cardiovascular causes (386 vs 502; HR, 0.74; 95% CI, 0.65-0.85; P < 0.001). This was driven by hospitalization (237 vs 326; HR, 0.70; 95% CI, 0.59-0.83) and cardiovascular death (227 vs 283; HR, 0.82; 95% CI, 0.69-0.98). There were also fewer total deaths observed in the dapagliflozin group (276 vs 329; HR, 0.83; 95% CI, 0.71-0.97). In subgroup analysis, only increasing NYHA class was associated with attenuated efficacy of dapagliflozin, although this finding might have been because of chance because no such interaction of outcomes was observed in the prespecified groups with increased baseline natriuretic peptides, those with lower EF, or those with previous hospitalization. Notably, the DAPA-HF study participants were well treated, with > 90% ACE/ARB and b-blocker use and 70% mineralocorticoid use at baseline. No treatment interaction with baseline medical therapy was seen, including with angiotensin receptor-neprilysin inhibitors (ARNI), whereas the primary end point showed statistical consistency with the overall trial (HR, 0.75; 95% CI, 0.50-1.10), which reinforces the efficacy of SGLT2 inhibitors in the setting of contemporary optimal medical therapy. Treatment with ARNI was used in approximately 11% of patients at baseline considering the timing of recruitment for this trial in relation to the publication of the Prospective Comparison of ARNi With ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial. The DAPA HF study included most participants (approximately 58%) who did not have concomitant diabetes. Subgroup analysis revealed a nearly identical reduction in the primary end point among nondiabetic patients (HR of 0.73 [95% CI, 0.60-0.88] in those without diabetes vs an HR of 0.75 [95% CI, 0.60-0.90] in those with diabetes). As such, SGLT2 inhibition with dapagliflozin has been shown for the first time to reduce morbidity and mortality in nondiabetic patients with HFrEF. Analyses of adverse events of interest in all patients, including volume depletion, adverse renal events, hypoglycemia, amputation, and ketoacidosis showed no increase in the dapagliflozin arm, reinforcing the favourable safety profile of this treatment in patients with HFrEF. Table 2 summarizes the results of large published clinical trials regarding the impact of SGLT2 inhibitors on heart failure and other cardiovascular outcomes.
Future directions
Although potential theories have been advanced to explain the benefits observed with SGLT2 inhibitors in preventing HF events, including reduction of preload via osmotic diuresis, lowering of afterload, alteration of myocardial energy substrate toward a more efficient glucose oxidation, modulation of renal sympathetic afferent tone, or direct reduction in myocardial mass, precise mechanisms associated with the clinical effect remain unknown. Further work in this area, including mechanistic studies in patients with established and treated HF, are needed and will be forthcoming. In addition, effective dosage of SGLT2 inhibitors is not yet known.[52]–[55] There are several other outstanding issues regarding the effect of SGLT2 inhibitors and prevention of HF events. First, additional data are required to clarify the relative effect of SGLT2 inhibitors according to LVEF and according to disease severity. Ongoing HF outcome studies should address existing evidence gaps across a range of EF values and clinical status. Accordingly, the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR-Reduced), the Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR PRESERVED), the Dapagliflozin Evaluation to Improve the Lives of Preserved Ejection Fraction Heart Failure (DELIVER) trials, and the Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial will further clarify the role of these agents in a broad spectrum of HF patients. For now, uncertainty remains regarding potential benefits of SGLT2 inhibitors for the following patient subgroups:
– Preserved or midrange LVEF;
– Acute HF;
– Isolated right HF or pulmonary hypertension; Advanced HF;
– Patients taking target doses of concomitant HF therapies including mineralocorticoid receptor antagonists, sacubitril-valsartan, ivabradine;
– Stage 4 and 5 kidney disease.
Recommendation
5. Updated
We recommend SGLT2 inhibitors, such as empagliflozin, canagliflozin or dapagliflozin, be used for treatment of patients with type 2 diabetes and athero-sclerotic cardiovascular disease to reduce the risk of HF hospitalization and death (Strong Recommendation, High-Quality Evidence).
6. New
We recommend SGLT2 inhibitors, such as dapagliflozin be used in patients with type 2 diabetes aged > 50 years with additional risk factors for atherosclerotic cardiovascular disease to reduce the risk of HHF (Strong Recommendation, High-Quality Evidence).
7. New
We recommend SGLT2 inhibitors, such as canagliflozin, be used in patients aged > 30 years with type 2 diabetes, and macroalbumineric renal disease, to reduce the risk of HF hospitalization and progression of renal disease (Strong Recommendation, High-Quality Evidence).
8. New
We recommend SGLT2 inhibitors, such as dapagliflozin be used in patients with mild to moderate HF due to reduced LVEF ( 40%) and concomitant type 2 diabetes, to improve symptoms and quality of life and to reduce the risk of hospitalization and cardiovascular mortality (Strong Recommendation, High-Quality Evidence).
9. New
We recommend SGLT2 inhibitors, such as dapagliflozin be used in patients with mild to moderate HF due to reduced LVEF ( 40%) and without concomitant diabetes, to improve symptoms and quality of life and to reduce the risk of hospitalization and cardiovascular mortality (Conditional Recommendation, High-Quality Evidence).
Values and Preferences
These recommendations place weight on the results of multiple large, randomized, placebo-controlled trials, which have tested 3 different SGLT2 inhibitors. These trials have conclusively shown that SGLT2 inhibitors reduce the risk of the incidence of HF, HF-related hospitalizations, and cardiovascular death in patients with type 2 diabetes and established cardiovascular disease. A strong recommendation for these agents in patients with type 2 diabetes with multiple risk factors for but without evidence of established cardiovascular disease is made despite the lack of reduction in cardiovascular death.
We considered the large relative benefit observed, the strong clinical priority for reduction of HF hospitalization, and the lack of any similar benefits associated with other antidiabetic agent in preventing incident HF. The strong recommendation for SGLT2 inhibitors for patients with type 2 diabetes and HFrEF reflects the large clinical benefit observed in this population and the current availability of these agents for patients with type 2 diabetes. A second, conditional recommendation for patients with HFrEF without concomitant diabetes is made because regulatory approval for this patient population in Canada is not currently available, and further data from the DAPA-HF trial regarding glucose status in those without diabetes might be clinically impactful. These medications are well tolerated and associated with an acceptable side effect profile within the clinical trials studied, with a low risk of genital mycotic infections, diabetic ketoacidosis or hypoglycemia, provided other antidiabetic medications are adjusted appropriately. The efficacy of this medication class is unproven at eGFR levels < 30 mL/min/1.73 m2. Table 3 summarizes practical issues surrounding initiation of SGLT2 inhibitors.
Practical Tip
It is worth emphasizing that SGLT2 inhibitors are currently contraindicated for patients with type 1 diabetes.
The most common adverse effect of this class of medications are genital mycotic infections (GMIs). Women (10%-15% risk), those with previous GMIs, and uncircumcised men are at highest risk. Typically, GMIs can be managed with antifungal drugs and do not require discontinuation of therapy.
SGLT2 inhibitors might result in temporary reduction of eGFR up to 15%, which generally resolves within 1-3 months. SGLT2 inhibitors have also been associated with acute kidney injury and increased monitoring is warranted in those at risk.
SGLT2 inhibitors do not cause hypoglycemia in the absence of concomitant insulin and/or secretagogue therapy. Background therapies might need to be adjusted to prevent hypoglycemia.
SGLT2 inhibitors should be held in the setting of concomitant dehydrating illness as part of “Sick Day” management. Patients should be educated on “Sick Day” management.[56]
These agents have been associated with diabetic ketoacidosis (incidence 0.1%). Patients might present with normal or only modestly elevated blood glucose level (< 14 mmol/L). On rare occasions, SGLT2 inhibitors might be associated with normal anion gap acidosis, which is best detected with measurement of serum ketones. Nonspecific symptoms associated with diabetic ketoacidosis include: shortness of breath, nausea, vomiting, abdominal pain, confusion, anorexia, excessive thirst, and lethargy.
Caution should be exercised when combining SGLT2 inhibitors, ARNI, and diuretics because of their concomitant effects to promote diuresis.
References
41. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89.
42. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545-59.
43. Group AS, Gerstein HC, Miller ME, et al. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 2011;364:818-28.
44. Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560-72.
45. Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360: 129-39.
46. MacDonald MR, Eurich DT, Majumdar SR, et al. Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested casecontrol study from the U.K. General Practice Research Database. Diabetes Care 2010;33:1213-8.
47. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373: 2117-28.
48. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26.
49. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med 2015;373: 232-42.
50. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial. Lancet 2015;385:2067-76.
51. Rosenstock J, Marx N, Neubacher D, et al. Cardiovascular safety of linagliptin in type 2 diabetes: a comprehensive patient-level pooled analysis of prospectively adjudicated cardiovascular events. Cardiovasc Diabetol 2015;14:57.
52. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375: 311-22.
53. Erdmann E, Charbonnel B, Wilcox RG, et al. Pioglitazone use and heart failure in patients with type 2 diabetes and preexisting cardiovascular disease: data from the PROactive study (PROactive 08). Diabetes Care 2007;30:2773-8.
54. Komajda M, McMurray JJ, Beck-Nielsen H, et al. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J 2010;31:824-31.
55. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007;356:2457-71.
56. Virani SA, Dent S, Brezden-Masley C, et al. Canadian Cardiovascular Society guidelines for evaluation and management of cardiovascular complications of cancer therapy. Can J Cardiol 2016;32:831-41.
