3. Integration of GLP-1RA or SGLT2i in Patients With T2D With or at Risk of atherosclerotic CVD
PICO 4: In patients with T2D and either atherosclerotic CVD (ASCVD) or high CV risk, what is the role of novel anti-hyperglycemic agents compared with placebo for reduction of a composite of CV death, nonfatal MI, or nonfatal stroke?
The initial trial that used empagliflozin published in 2015, through to the most recent trial that used efpeglenatide published in 2021 were evaluated in detail in the accompanying systematic review and meta-analysis.[7] Table 1 shows that MACE was reduced similarly by both classes with a relative risk reduction of 12%-14%. These classes were also associated with similar relative risk reductions in all-cause (12%-15%) and CV mortality (13%-15%). Reduction in nonfatal MI was noted only with SGLT2i but the effect was modest (10% relative risk reduction). Because this effect was not statistically different from the neutral effects on nonfatal MI associated with the GLP-1RA class, we make no recommendation on the basis of this end point. The SGLT2i class showed significant relative risk reduction for the prevention of the composite kidney outcomes (35%) and for hospitalization for HF (32%) compared with placebo and superior to GLP-1RA. As noted previously, currently we do not have any large clinical trials for the treatment of HF or CKD in patients with or without T2D using GLP-1RA. Finally, the important but less common outcome of nonfatal stroke was reduced with GLP-1RA, particularly in the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) 6 trial (semaglutide once weekly injection) and Researching Cardiovascular Events With a Weekly Incretin in Diabetes (REWIND; dulaglu- tide) trials.[9],[46],[47] The systematic review and meta-analysis (Table 1) indicate a relative risk reduction of nonfatal stroke of 16% associated with use of GLP-1RA.
RECOMMENDATION
4. In adults with T2D and either established ASCVD or multiple risk factors for ASCVD, we recommend use of:
- GLP-1RA or SGLT2i to reduce the risk of all-cause or CV mortality or MACE (Strong Recommendation; Moderate-Quality Evidence),
- SGLT2i to reduce the risk of hospitalization for HF or the composite of significant decline in eGFR, progression to end-stage kidney disease or kidney death (Strong Recommendation; Moderate-Quality Evidence),
- GLP-1RA to reduce the risk of nonfatal stroke (Strong Recommendation; Moderate-Quality Evidence).
Practical tip
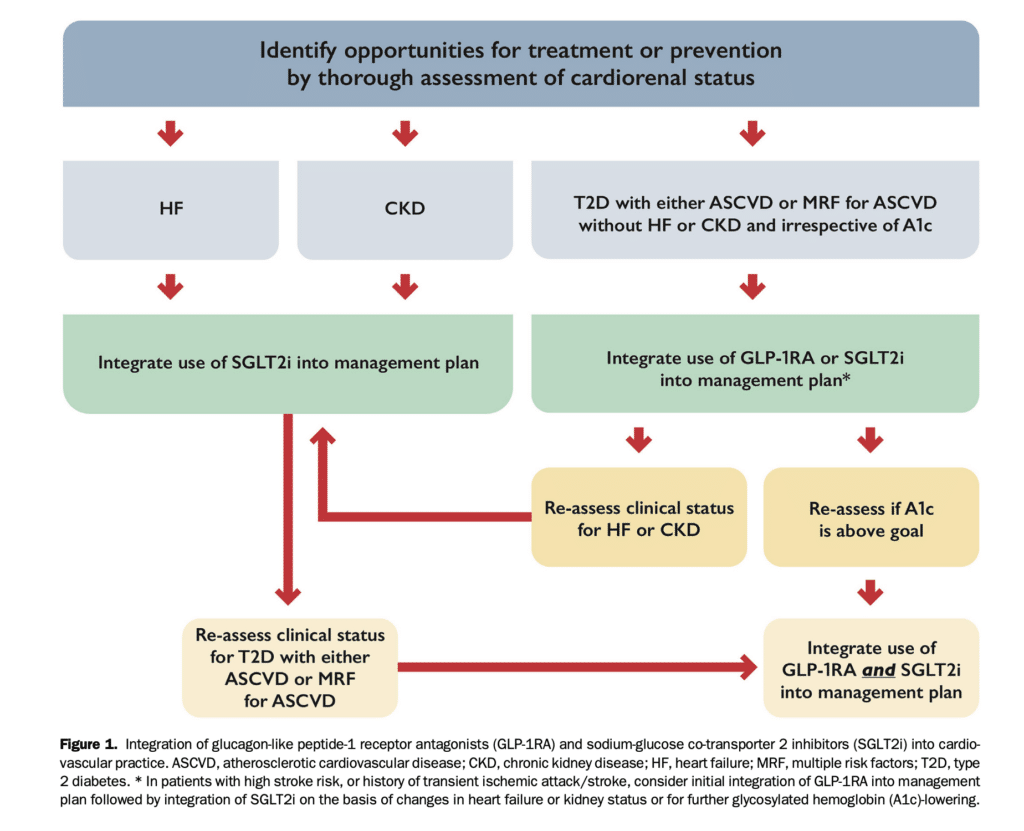
A combination of SGLT2i and GLP-1RA might theoretically improve cardiorenal benefits in patients with T2D and either ASCVD or multiple risk factors for ASCVD whose A1c remains suboptimal despite initial treatment with only one of these agents or if clinical status changes (e.g., new onset HF or CKD; Fig. 1).
General discussion
The principles of pharmacotherapy for patients with T2D have been thoroughly reviewed by Diabetes Canada.[3],[4]Achievement of target glucose levels, especially in the early years after T2D diagnosis, reduces the incidence and progression of microvascular complications and, in the long term (more than 10 years), is associated with reduced CV outcomes.[48-51] In parallel with achieving the A1c goal, it is also recommended that GLP-1RA or SGLT2i be included for patients with T2D with or at high risk of ASCVD to reduce cardiorenal risk, irrespective of A1c. Thus, substitution of (replacing rather than adding) an agent with cardiorenal benefit might be appropriate if people are at or near A1c target.
The choice of initial pharmacotherapy has emerged as an area of uncertainty in patients with newly diagnosed T2D who have or are at risk of ASCVD. Although most guidelines continue to recommend metformin as first-line anti-hyperglycemic therapy, the European Society of Cardiology[52] recommends that GLP-1RA or SGLT2i should be first-line therapy in individuals with ASCVD or at high or very high CV risk. Although there have been no specific trials to show cardiorenal benefit for GLP-1RA or SGLT2i when used as first-line therapy or as monotherapy or in newly diagnosed T2D, the benefit seen in the CV outcome trials has not been found to vary with the duration of diabetes, suggesting that similar benefits might be seen early in the course of disease.4 The benefits are also not dependent on the presence of metformin.[8],[53-55] Therefore, the inclusion of GLP-1RA or SGLT2i at the time of diagnosis of T2D in patients with ASCVD or multiple risk factors is a reasonable option and aligns with the views of Diabetes Canada.[3],[4] In addition, the traditional role of metformin in the early management of T2D is not always appropriate if not tolerated or contraindicated (eg, eGFR < 30 mL/min/1.73 m2). In HF, including HFrEF and HFpEF, SGLT2i used in addition to other evidence-based HF therapies but without treatment with metformin were shown to improve major HF-related outcomes and quality of life within a short period of time after initiation of therapy. Moreover, benefit was seen in patients with and those without T2D.[8],[53-55]
SGLT2i reduce hospitalization for HF[15],[20] and reduce progression of nephropathy[15],[19] in persons with T2D and CV risk factors only; benefits to reduce MACE or mortality, at least within the short-term duration of the trials to date, are less certain.[56],[57] Conversely, in such patients, GLP-1RA seem to reduce MACE,[9],[12],[13] a factor that might help selection between SGLT2i or GLP-1RA for A1c reduction. Our analyses (Table 1) indicate that the reduction of nonfatal stroke is strongest for GLP-1RA, which might also factor into the initial choice of classes.
Opinions vary about whether beneficial effects are general to a class or specific to individual agents. Although network meta-analyses have attempted to provide comparisons of specific SGLT2i or GLP-1RA, no head-to-head trials are currently available that help differentiate between medications within either of these 2 classes.[56],[58] Consequently, the writing group consensus emphasizes class effects but recognizes that some outcomes have been associated with specific agents (Table 3). Using both classes together to achieve glycemic targets when needed appears to be a reasonable option. However, it is not known whether additional cardiorenal benefit can be expected by combining both classes, although the potential mechanisms might be complementary. The most recent GLP-1RA CV outcome trial showed similar benefit whether the patient was using SGLT2i or not.[10],[59] Finally, an individualized approach to therapy should also weigh the individual’s preferences, costs and coverage, side effect profile, consideration of kidney function and glucose-lowering efficacy, desire for weight loss, and comorbidities such as frailty. Although diminished kidney function attenuates the glucose-lowering effects of SGLT2i, cardiorenal protection is maintained with an eGFR > 20-25 mL/min/1.73 m2.[21],[22],[36]
References
2. Mancini GBJ, Cheng AY, Connelly K, et al. CardioDiabetes: core competencies for cardiovascular clinicians in a rapidly evolving era of type 2 diabetes management. Can J Cardiol 2018;34:1350-61.
3. Lipscombe L, Butalia S, Dasgupta K, et al. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 2020;44:575-91.
4. Senior PA, Houlden RL, Kim J, et al. Pharmacologic glycemic man- agement of type 2 diabetes in adults: 2020 update – the user’s guide. Can J Diabetes 2020;44:592-6.
7. Ali MU, Mancini GBJ, Fitzpatrick-Lewis D, et al. The effectiveness of sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists on cardiorenal outcomes: systematic review and meta- analysis. Can J Cardiol 2022;38:1201-10.
8. Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomized placebo-controlled trial. Lancet 2018;392:1519-29.
9. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet 2019;394:121-30.
10. Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med 2021;385: 896-907.
12. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and car- diovascular outcomes in type 2 diabetes. N Engl J Med 2016;375: 311-22.
13. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375: 1834-44.
19. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347-57.
21. Packer M, Anker SD, Butler J, et al. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR- Reduced Trial. J Am Coll Cardiol 2021;77:1381-92.
22. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451-61.
36. Packer M, Butler J, Zannad F, et al. Empagliflozin and major renal outcomes in heart failure. N Engl J Med 2021;385:1531-3.
46. Leiter LA, Bain SC, Hramiak I, et al. Cardiovascular risk reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, age, and baseline CV risk profile in the SUSTAIN 6 trial. Cardiovasc Diabetol 2019;18:73.
47. Goldenberg RM, Cheng AYY, Fitzpatrick T, Gilbert JD, Verma S, Hopyan JJ. Benefits of GLP-1 (glucagon-like peptide 1) receptor agonists for stroke reduction in type 2 diabetes: a call to action for neurologists. Stroke 2022;53:1813-22.
48. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood- glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837-53.
49. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577-89.
50. ACCORD Study Group. Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care 2016;39: 701-8.
51. Mancini GBJ, Maron DJ, Hartigan PM, et al. Lifestyle, glycosylated hemoglobin A1c, and survival among patients with stable ischemic heart disease and diabetes. J Am Coll Cardiol 2019;73:2049-58.
52. Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC guidelines on dia- betes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255-323.
53. Inzucchi SE, Fitchett D,Jurisic-Erzen D,et al. Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose-lowering therapy? Diabetes Obes Metab 2020;22:631-9.
54. Ferrannini G, Gerstein H, Colhoun HM, et al. Similar cardiovascular outcomes in patients with diabetes and established or high risk for cor- onary vascular disease treated with dulaglutide with and without baseline metformin. Eur Heart J 2021;42:2565-73.
55. Crowley MJ, McGuire DK, Alexopoulos AS, et al. Effects of liraglutide on cardiovascular outcomes in type 2 diabetes patients with and without baseline metformin use: post hoc analyses of the LEADER trial. Diabetes Care 2020;43:e108-10.
56. Palmer SC, Tendal B, Mustafa RA, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ 2021;372:m4573.
57. Wright AK, Carr MJ, Kontopantelis E, et al. Primary prevention of cardiovascular and heart failure events with SGLT2 inhibitors, GLP-1 receptor agonists, and their combination in type 2 diabetes. Diabetes Care 2022;45:909-18.
58. Duan XY, Liu SY, Yin DG. Comparative efficacy of 5 sodium glucose cotransporter 2 inhibitor and 7 glucagon-like peptide 1 receptor agonists.
59. Lam CSP, Ramasundarahettige C, Branch KRH, et al. Efpeglenatide and clinical outcomes with and without concomitant sodium-glucose co-transporter-2 inhibition use in type 2 diabetes: exploratory analysis of the AMPLITUDE-O trial. Circulation 2022;145:565-74.
