3. Sinus Node Inhibition
Resting heart rate independently predicts CV events, including HHF and death.[52]–[54] Studies have shown that the effect of elevated heart rate on outcomes becomes apparent within 30 days of discharge from hospital.[55] In systematic reviews it has been postulated that a major contributor to the benefits of β-blocker therapy in patients with HFrEF might be their rate-lowering effect.[56]–[58]
Ivabradine selectively inhibits the depolarizing Ifcurrent in the sinus node. It thus requires sinus rhythm to provide its pharmacological effect. In contrast to β-blockers, ivabradine decreases heart rate without lowering BP or myocardial contractility.[59] The Systolic Heart Failure Treatment With the IfInhibitor Ivabradine Trial (SHIFT) trial addressed the use of ivabradine in ambulatory patients with chronic symptomatic HFrEF.[60] The SHIFT trial design, inclusion criteria, and results have been discussed previously in the 2017 comprehensive guideline update.[1] In this trial, there was an 18% reduction in the primary outcome of CV death or HHF favouring ivabradine compared with placebo, which was largely driven by a reduction in HHF (relative risk reduction, 26%). In the prespecified subgroup of patients with resting heart rate > 77 bpm, ivabradine exerted a greater effect on outcome reduction including the primary end point (HR, 0.76 [95% CI 0.68-0.85]; P < 0.0001), all-cause mortality (HR, 0.83 [95% CI 0.72-0.96]; P 1⁄4 0.0109), and CV mortality (HR, 0.83 [95% CI 0.71-0.97]; P 1⁄4 0.0166).[61] In the 685 patients not taking β-blockers at baseline, ivabradine reduced the primary end point with a HR of 0.68 (95% CI 0.52-0.88).
Studies have shown that most titration of β-blockade occurs early in the course of treatment, with most of the heart rate reduction occurring at < 50% of target dose.[62],[63] With further titration, there is a diminishing effect on heart rate, leaving approximately 10%-15% of patients with residual heart rate > 70 bpm after β-blocker titration.[64],[65] Beyond chronic ambulatory HF, small studies have shown that the additional use of ivabradine with a β-blocker is safe and well tolerated in hospital settings.[66]–[69]
Recommendation
15. We recommend that ivabradine be used for patients with HFrEF and symptoms despite treatment with GDMT, a resting heart rate ≥ 70 bpm, and sinus rhythm for the prevention of CV death and HF hospitalization (Strong Recommendation; High-Quality Evidence).
Values and Preferences
High value is placed on reducing the risk of CV death and HHF when ivabradine is used as adjunctive therapy with standard HF medication treatments in a selected HFrEF population. Differing criteria for heart rate eligibility have been approved by various regulatory authorities ranging from 70-77 bpm, although the trial entry criteria was 70 bpm.
Practical Tip
Ivabradine has no direct effect on BP, myocardial contractility, or renal function and as such is well tolerated in patients who are unable to initiate or titrate β-blockers for these reasons.
Ivabradine may be considered for patients with either stable or decompensated chronic HFrEF who are intolerant of β-blockers, with a resting heart rate in sinus rhythm of > 70 bpm.
Typical reductions in resting sinus heart rate after treatment with β-blockers range from 10-15 bpm, with little change (< 5 bpm) between low and high doses. This consideration might assist in the decision to use further medications for sinus heart rate control.
Ivabradine is well tolerated in older adults and can be initiated at 2.5 mg twice daily.
Ivabradine should be avoided in patients with advanced liver disease.
sGC stimulators
Worsening HF and HHF portend a poor prognosis and are associated with increased risk of mortality and recurrent hospitalization. The initial posthospitalization phase is the highest risk period for adverse events and represents an opportunity for the clinician to optimize HF care.[70] Pharmacological therapies targeted at this vulnerable phase of the patient journey as a strategy to improve longer-term outcomes have been evaluated in recent clinical trials.[71],[72]
sGC stimulators, such as vericiguat, directly enhance cyclic guanylate monophosphate (GMP) production and also enhance endogenous sGC sensitivity to nitric oxide. This results in a cascade of adaptive effects on the heart, blood vessels, and kidneys, providing the physiological rationale for their use in patients with HF.
In the Vericiguat Global Study in Subjects With Heart Failure With Reduced Ejection Fraction (VICTORIA) trial the efficacy and safety of vericiguat compared with standard of care was evaluated in patients with advanced functional symptoms, an LVEF < 45% and a worsening HF event characterized by HHF or elevated natriuretic peptide levels.[71] Notably, patients with an eGFR < 15 mL/min/m2 and systolic BP of < 100 mm Hg were excluded. Study participants receiving optimal guideline-based HF therapies were randomized to placebo or vericiguat and followed for an average of 11.8 months. The primary combined end point of CV death or first HHF was significantly lower (HR, 0.90 [95% CI 0.82-0.98]; P 1⁄4 0.019) in the vericiguat group and this was driven primarily by a reduction in hospitalization rather than CV death. Of note, the secondary end point of total HHF was also decreased in the vericiguat group (HR, 0.91 [95% CI 0.84-0.99]; P 1⁄4 0.023). From a safety perspective, there was more hypotension in the vericiguat group but this did not contribute to renal dysfunction, despite the relatively low eGFR cutoff for enrollment.
Intention to treat subgroup analysis of the combined primary end point showed that vericiguat provided benefit across most clinically relevant subgroups with exception of those with very high NT-proBNP values at baseline (> 8000 pg/mL).[73]
Recommendation
16. We recommend that vericiguat, an oral sGC stimulator, be considered in addition to optimal HF therapies for HFrEF patients with worsening symptoms and HHF in the past 6 months, to reduce the risk of subsequent HF hospitalization (Conditional Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation places value on the use of an additional medication to reduce the risk of HHF in a high-risk patient population that experiences high rates of hospitalization and mortality despite the relatively modest relative benefits observed in the VICTORIA trial.
A conditional recommendation is provided because vericiguat has not yet been approved for this indication in Canada.
Practical Tip
Subgroup analysis from the VICTORIA trial suggests that clinical response to vericiguat might be attenuated in patients with very elevated natriuretic peptide levels.
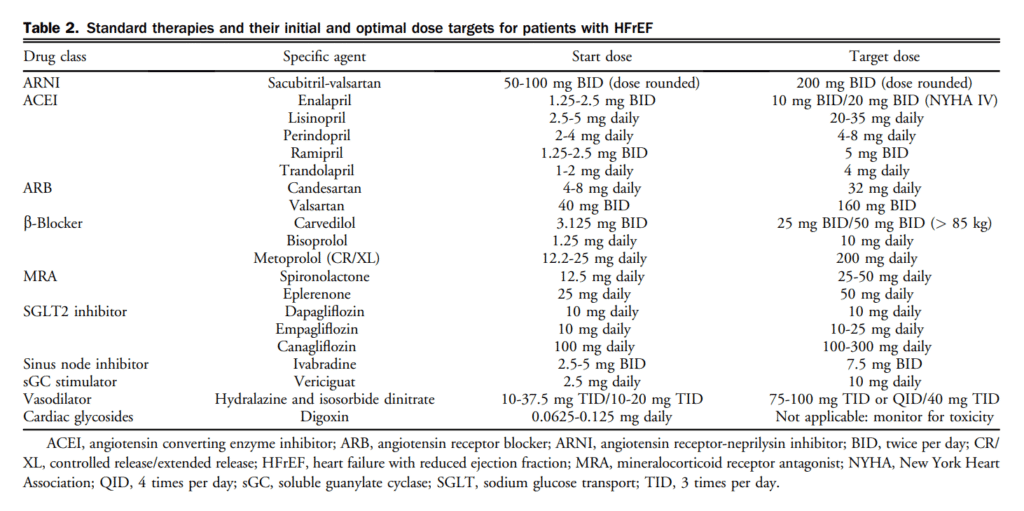
Digoxin
The Digitalis Investigation Group (DIG) trial enrolled 6800 patients with HF and a LVEF ≤ 45%. The primary end point was mortality, and the mean follow-up was 37 months. Patients were randomized to digoxin (median dose, 0.25 mg/d) or placebo. Fifty-four percent of participants had NYHA class II symptoms and 94% were treated with an ACEI. There was no difference in all-cause mortality between groups. There were fewer patients hospitalized for worsening HF in the digoxin group. Suspected digoxin toxicity was higher in the digoxin group.[74]
Recommendation
17. We suggest digoxin be considered in patients with HFrEF and atrial fibrillation, with poor control of ventricular rate and/or persistent symptoms despite optimally tolerated β-blocker therapy, or when β-blockers are not tolerated, in the setting of chronic HF, new onset HF, or HF hospitalization (Weak Recommendation; Low-Quality Evidence).
18. We suggest digoxin be considered in patients with HFrEF in sinus rhythm who continue to have moderate to severe symptoms despite appropriate doses of GDMT to relieve symptoms and reduce hospitalizations (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendations place a high value on the understanding that the role of cardiac glycosides in patients with HFrEF remains controversial in light of evolving contemporary HF therapy.
A subsequent systematic review of 13 studies (which included the DIG trial) showed similar results. None of these studies provide meaningful insight into the relative benefit, or harm, of digoxin in light of contemporary HFrEF therapy. There has been substantial use of digoxin as background therapy in the current era of HFrEF landmark trials with no apparent change in outcomes stratified according to baseline digoxin use.[75]
Practical Tip
Serum concentrations of digoxin < 1.2 ng/mL are associated with less treatment-related morbidity. Nonetheless, routine digoxin levels are not required other than to assess for digoxin toxicity. Digoxin levels should not be used to guide chronic therapy and titrating to digoxin levels has not been tested in clinical trials.
Digoxin can cause atrial and ventricular arrhythmias particularly in the presence of hypokalemia and/or worsening renal function and levels should be monitored accordingly.
In patients receiving digoxin, serum potassium and creatinine should be measured with in digoxin or diuretic dose, the additional use or discontinuation of an interacting drug, or during a dehydrating illness, to reduce the risk of digoxin toxicity. Patients with reduced or fluctuating renal function, older patients, those with low body weight, and women are at increased risk of digoxin toxicity and might require more frequent monitoring including digoxin levels.
Among hospitalized older patients with HFrEF who are receiving guideline-directed medical therapies, discontinuation of preadmission digoxin therapy might have deleterious effects.[76]
Hydralazine and isosorbide dinitrate
The combination of hydralazine and isosorbide dinitrate (H-ISDN) has had a role in the management of HFrEF since the 1980s. The first large-scale trial of this therapy predated landmark studies of RASi and β-blockers. In Vasodilator in Heart Failure Trial (V-HeFT) the effect of H-ISDN, prazosin, and placebo were compared in an HFrEF patient population. Mortality was reduced among patients treated with H-ISDN with a relative risk reduction of 34% at 2 years (P 1⁄4 0.028).[77] Compared with enalapril, treatment with H-ISDN provided less mortality reduction after a mean of 2.5 years (32.8% vs 38.2%; P 1⁄4 0.016) and no difference in hospitalizations.[78]
In the African-American Heart Failure Trial (A-HeFT), H-ISDN was investigated as used in addition to optimal therapy in self-identified black patients with HFrEF and NYHA class III/IV symptoms. Black patients were specifically evaluated in this trial because they are known to have reduced activity of the renin-angiotensin system. A total of 1050 black patients were randomized to H-ISDN or placebo, in addition to standard of care, and followed for a mean of 10 months. The study was terminated early because of higher mortality in the placebo group. The primary outcome was a weighted score, but individual components of the outcome showed a difference favouring H-ISDN for all-cause mortality, first HHF, and change in quality of life score.[79]
Recommendation
19. We recommend that H ISDN be considered for treatment of patients with HFrEF who are unable to tolerate an ACEI, ARB, or ARNI because of hyperkalemia, renal dysfunction, or other contraindications, in the following settings:
i. Chronic HF (Strong Recommendation, Moderate-Quality Evidence);
ii. New-onset HF (Weak Recommendation, Low-Quality Evidence); and
iii. HF hospitalization (Weak Recommendation, Low-Quality Evidence).
20. We recommend that H ISDN treatment be considered in addition to standard GDMT at appropriate doses for black patients with HFrEF and advanced symptoms (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
There is limited high-quality clinical trial evidence in the modern era on which to base an H-ISDN recommendation. Adverse effects related to H-ISDN are frequent, limit up-titration, and lead to discontinuation in a significant proportion of patients. Every effort should be made to use ARNI (or alternatively ACEI/ARB) therapy including initiating at a low dose and/or rechallenging patients who have experienced adverse events/intolerability before changing to H-ISDN.
Practical Tip
Renal dysfunction warranting a trial of H-ISDN includes those who have a significant change in creatinine from baseline with ACEI/ARB/ARNI therapy that persists despite modification of dose, rechallenge, and/or removal of other potentially nephrotoxic agents. It may also be considered in those with a serum creatinine > 220 mmol/L who experience significant worsening in renal function with the use of ACEI/ARB/ARNI therapy, or if the risk of these agents (eg, potential for worsening renal function requiring renal replacement therapy) is thought to outweigh benefits.
A trial of H-ISDN might be warranted in patients with persistent hyperkalemia (K > 5.5 mmol/L) despite dietary intervention, dose reduction of ACEI/ARB/ARNI, and removal of other agents known to increase potassium levels.
Nitrates alone might be useful to relieve orthopnea, paroxysmal nocturnal dyspnea, exercise-induced dyspnea, or angina in patients when used as tablet, spray, or transdermal patch, but continuous (ie, around the clock) use should generally be avoided because most patients will develop tolerance. It should be noted that use of nitrates or hydralazine alone has not been shown to improve HF outcomes.
References
51. Canadian Heart Failure Society: Practical approach to SGLT2 inhibitors for treatment of cardiovascular disease. Available at: https://heartfailure.ca/sites/default/files/chfs_practical_approach_algorithm_sglt2i_0.pdf. Accessed February 9, 2021.
52. Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J 2005;26:967-74.
53. Fox K, Ford I, Steg PG, et al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 2008;372:817-21.
54. Docherty KF, Shen L, Castagno D, et al. Relationship between heart rate and outcomes in patients in sinus rhythm or atrial fibrillation with heart failure and reduced ejection fraction. Eur J Heart Fail 2020;22:528-38.
55. Laskey WK, Alomari I, Cox M, et al. Heart rate at hospital discharge in patients with heart failure is associated with mortality and rehospitalization. J Am Heart Assoc 2015;4:e001626.
56. McAlister FA, Wiebe N, Ezekowitz JA, Leung AA, Armstrong PW. Meta-analysis: beta-blocker dose, heart rate reduction, and death in patients with heart failure. Ann Intern Med 2009;150:784-94.
57. Komajda M, Hanon O, Hochadel M, et al. Contemporary management of octogenarians hospitalized for heart failure in Europe: Euro Heart Failure Survey II. Eur Heart J 2009;30:478-86.
58. Flannery G, Gehrig-Mills R, Billah B, Krum H. Analysis of randomized controlled trials on the effect of magnitude of heart rate reduction on clinical outcomes in patients with systolic chronic heart failure receiving beta-blockers. Am J Cardiol 2008;101:865-9.
59. DiFrancesco D, Borer JS. The funny current: cellular basis for the control of heart rate. Drugs 2007;67(suppl 2):15-24.
60. Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875-85.
61. Bouabdallaoui N, O’Meara E, Bernier V, et al. Beneficial effects of ivabradine in patients with heart failure, low ejection fraction, and heart rate above 77 b. m. ESC Heart Fail 2019;6:1199-207.
62. Bohm M, Borer J, Ford I, et al. Heart rate at baseline influences the effect of ivabradine on cardiovascular outcomes in chronic heart failure: analysis from the SHIFT study. Clin Res Cardiol 2013;102:11-22.
63. Bristow MR, Gilbert EM, Abraham WT, et al. Carvedilol produces dose related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators. Circulation 1996;94:2807-16.
64. Metra M, Torp-Pedersen C, Swedberg K, et al. Influence of heart rate, blood pressure, and beta-blocker dose on outcome and the differences in outcome between carvedilol and metoprolol tartrate in patients with chronic heart failure: results from the COMET trial. Eur Heart J 2005;26:2259-68.
65. Das D, Savarese G, Dahlstrom U, et al. Ivabradine in heart failure: the representativeness of SHIFT (Systolic Heart Failure Treatment With the IF Inhibitor Ivabradine Trial) in a broad population of patients with chronic heart failure. Circ Heart Fail 2017;10:e004112.
66. Hidalgo FJ, Anguita M, Castillo JC, et al. Effect of early treatment with ivabradine combined with beta-blockers versus beta-blockers alone in patients hospitalised with heart failure and reduced left ventricular ejection fraction (ETHIC-AHF): a randomised study. Int J Cardiol 2016;217:7-11.
67. Bagriy AE, Schukina EV, Samoilova OV, et al. Addition of ivabradine to beta-blocker improves exercise capacity in systolic heart failure patients in a prospective, open-label study. Adv Ther 2015;32:108-19.
68. Barilla F, Pannarale G, Torromeo C, et al. Ivabradine in patients with ST-elevation myocardial infarction complicated by cardiogenic shock: a preliminary randomized prospective study. Clin Drug Investig 2016;36:849-56.
69. Mert KU, Mert GO, Morrad B, et al. Effects of ivabradine and beta-blocker therapy on dobutamine-induced ventricular arrhythmias. Kardiol Pol 2017;75:786-93.
70. Solomon SD, Dobson J, Pocock S, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 2007;116:1482-7.
71. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med 2020;382: 1883-93.
72. Teerlink JR, Diaz R, Felker M, et al. Cardiac myosin activation with omecamtiv mecarbil in systolic heart failure. N Engl J Med 2021;384:105-16.
73. Ezekowitz JA, O’Connor CM, Troughton RW, et al. N-terminal proB-type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail 2020;8:931-9.
74. Digitalis Investigation Group: The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525-33.
75. Hood WB Jr, Dans AL, Guyatt GH, Jaeschke R, McMurray JJ. Digitalis for treatment of heart failure in patients in sinus rhythm. Cochrane Database Syst Rev 2014;4:CD002901.
76. Malik A, Masson R, Singh S, et al. Digoxin discontinuation and outcomes in patients with heart failure with reduced ejection fraction. J Am Coll Cardiol 2019;74:617-27.
77. Cohn JN, Archibald DG, Ziesche S, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. N Engl J Med 1986;314:1547-52.
78. Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991;325:303-10.
79. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004;351:2049-57.
