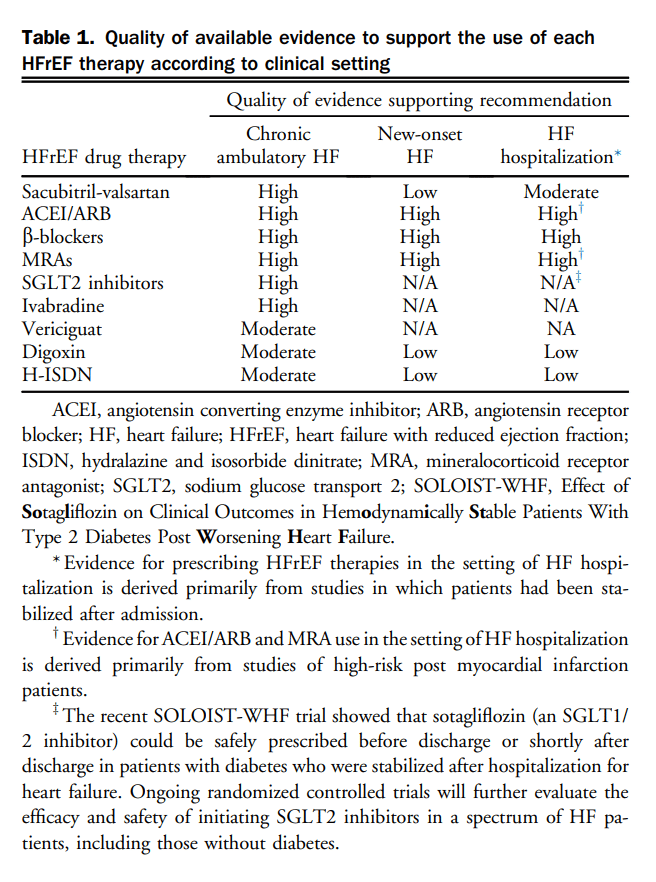
2. Standard Therapies
On the basis of new and emerging evidence for the pharmacologic treatment of HFrEF, updated treatment recommendations are provided herein. In the current era, patients with HFrEF should treated with 4 standard therapies, in the absence of contraindications, each representing a different class of medication with unique mechanism of action. Placing a high priority on reducing cardiovascular (CV) mortality and hospitalization for HF (HHF) in most patients, these medications include: (1) an ARNI, either as first-line therapy or switching from an angiotensin converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB); (2) a β-blocker; (3) a mineralocorticoid receptor antagonist (MRA); and (4) an SGLT2 inhibitor. Specific recommendations for each class of therapy, including the clinical settings in which these treatments may be prescribed, are outlined in detail in the sections that follow. Beyond these standard therapies, additional medications benefit important subgroups of patients with HFrEF, and should be initiated and titrated where indicated. In particular, the role and clinical settings for prescription of ivabradine (sinus node inhibitor), vericiguat (sGC stimulator), digoxin, and hydralazine/nitrates are discussed under their respective headings. Table 1 highlights the quality of available evidence to support the use of each HFrEF therapy according to clinical setting.
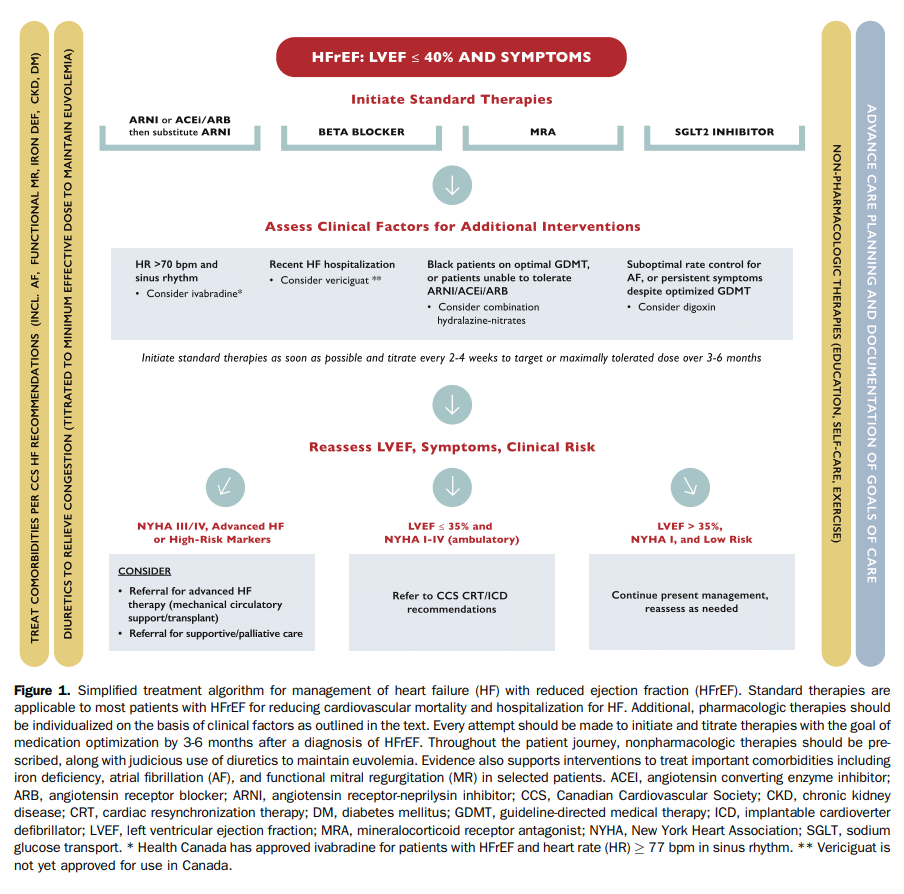
A simplified, HFrEF treatment algorithm is illustrated in Figure 1. Recognizing that any such algorithm cannot address all of the nuances and multiple considerations underpinning individualized HFrEF management in the current era, the approach presented places value on pragmatic considerations for most patients. Depending on the clinical practice environment, initiation and titration of standard therapies should be embraced by nonspecialists, whereas additional pharmacologic and interventional considerations might warrant input from specialists.
It is worth noting that the “algorithm” in Figure 1 has been informed by best available evidence and the consensus of the Primary Panel, but to date, there is no proven superior approach to medication initiation and titration. For example, on the basis of clinical characteristics, it might be preferable to titrate doses of different classes of medications simultaneously (“in-parallel” approach), rather than fully titrate one medication class before initiating an additional agent (“strict sequential” approach). Although newer medication classes such as ARNI and SGLT2 inhibitors were evaluated in patients with high background use of β-blockers, MRAs, and ACEIs or ARBs, there is currently no Primary Panel consensus endorsing a fixed sequence for medication prescription for patients with HFrEF. There is, however, consensus that all 4 classes of therapies should be used in patients with HFrEF and detailed evidence for each specific drug class is presented in the appropriate section.
Recommendation
1. We recommend that in the absence of contraindications, patients with HFrEF be treated with combination therapy including 1 evidence-based medication from each of the following categories:
a. ARNI (or ACEI/ARB);
b. β-blocker;
c. MRA; and
d. SGLT2 inhibitor.
(Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
High value is placed on prescribing a combination of individual therapies that reduce CV mortality and HHF in well conducted randomized controlled trials. Medications such as ARNI and SGLT2 inhibitor have clinical benefits in patients treated with ACEIs or ARBs, β-blockers, and MRAs as background therapy. The complementary mechanisms of action of these agents in patients with HFrEF provides further rationale for a multidrug approach.
Preference is given to the use of pharmacotherapy in patients with established HFrEF regardless of symptom severity.
The Committee acknowledges lack of evidence favouring one particular titration strategy for guideline directed medical therapy (GDMT) over another.
Practical Tip
The approach to initiation and titration of standard therapies should be directed by clinical and other patient factors including hemodynamic status, renal function, access to medication, adherence, anticipated side effects and tolerability, and patient preference.
Every attempt should be made to titrate medications as soon as feasible after the diagnosis. It is reasonable to aim for titration of all standard therapies concurrently to target doses, or maximally tolerated doses, within 3-6 months from diagnosis.
Because of the superiority of ARNI over ACEIs or ARBs in the setting of HFrEF, prescribing ARNI as first-line therapy or before full titration of ACEIs/ARBs might facilitate more rapid optimization of GDMT.
If a drug with proven mortality or morbidity benefits does not appear to be tolerated (eg, low blood pressure [BP], low heart rate, or renal dysfunction), concomitant drugs (eg, diuretics) with less proven benefit should be carefully reevaluated to determine whether their dose can be reduced or the drug discontinued.
GDMT for HFrEF should be continued at the usual dose during acute intercurrent illness unless they are not tolerated or could potentially worsen severity of illness. Whenever possible, GDMT withheld during a hospitalization should be restarted before discharge.
In the event of a life-threatening complication, GDMT maybe discontinued abruptly, but generally, if there is concern about their use, the dose should be decreased by one-half, and the patient should be reassessed. If the dose is reduced, the previous tolerated dose should be resumed as soon as safely possible.
If symptomatic hypotension persists with GDMT, consider separating the administration of the dose from RECOMMENDATION the timing of other medications that could also lower BP.
Recommendation
2. We recommend preferentially use of drugs at target doses that have been proven to be beneficial in clinical trials as optimal medical therapy. If these doses cannot be achieved, the maximally tolerated dose is acceptable (Table 2; Strong Recommendation; High Quality Evidence).
ARNI
Registry data continue to identify suboptimal initiation and titration of goal-directed medical therapy in patients with ambulatory HF.[4] Thus, HHF represents an ideal time to recalibrate, and optimize the treatment plan by initiating GDMT. ARNI therapy is now a well established treatment recommendation in patients with chronic HFrEF who have been previously exposed to either ACEIs or ARBs. The multicentre, randomized, double-blind, parallel group, active controlled study to evaluate the efficacy and safety of LCZ696 compared to enalapril on morbidity and mortality in patients with chronic HFrEF (Prospective Comparison of ARNi With ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure [PARADIGM-HF]) trial[5] showed superior efficacy of ARNI therapy over enalapril in chronic HF patients already receiving maximally tolerated dose of a renin-angiotensin system inhibitor (RASi). More recently, the safety and efficacy of this strategy has been explored in patients hospitalized with acute HF, including de novo HF, with or without previous exposure to RASi. The Comparison of Pre-discharge and Post-Discharge Treatment Initiation With LCZ696 in Heart Failure Patients With Reduced Ejection Fraction Hospitalized for an Acute Decompensation Event (TRANSITION) study[6] was an open-label multicentre randomized controlled trial of 1002 patients, which showed the safety of initiating ARNI in patients with left ventricular ejection fraction (LVEF) 40% admitted to hospital with decompensated HF (median 7 days from admission) compared with initiation of ARNI therapy after discharge (median 10 days from admission). There was no difference in the proportion of patients who achieved maximum dose of sacubitril-valsartan at 10 weeks of follow-up (45.4% vs 50.7%; relative risk [RR] 0.90 [95% CI 0.79-1.02] in the pre and post-discharge initiation groups, respectively). Similarly, there was no difference in the proportion of patients tolerating any dose of drug at 10 weeks with either strategy (86.0% vs 89.6%; RR, 0.96 [95% CI 0.92-1.01]). In a recent TRANSITION substudy 286 patients with de novo HF were compared with 705 patients with established HF and those with newly diagnosed HF were shown to be more likely to achieve target dose of sacubitril-valsartan at 10 weeks (56% vs 45%; RR, 1.30 [95% CI 1.12-1.52]; P < 0.001) with fewer serious adverse reactions.[7] Patients with de novo HFrEF who started ARNI therapy had a greater decrease in N-terminal pro hormone brain natriuretic peptide (NT-proBNP) and lower rates of rehospitalization without compromising up-titration of other guideline-directed HF therapies.
Further support for initiating ARNI as first-line HFrEF therapy in de novo or RASi-naive patients comes from the Comparison of Sacubitril/Valsartan Versus Enalapril on Effect on Nt-Pro-Bnp in Patients Stabilized From an Acute Heart Failure Episode (PIONEER-HF) trial,[8] and its open-label extension study.[9] In this double-blind randomized controlled trial, in-hospital initiation of sacubitril-valsartan was compared with enalapril in 881 HFrEF patients hospitalized with HF. Notably, one-third of patients enrolled did not have a history of HF and just more than half had no previous ACEI or ARB use. In-hospital initiation of sacubitril-valsartan resulted in a significantly greater proportional reduction in NT-proBNP compared with enalapril at weeks 4 and from baseline (mean time-averaged change in NT-proBNP, 46.7% vs 25.3%). This change was consistent across all subgroups, including those without previous HF and those who were RASi-naive. In the open-label extension, the clinical course of patients in the PIONEER-HF trial was evaluated for those who initiated sacubitril-valsartan treatment in-hospital as well as for those who switched from enalapril to sacubitril-valsartan treatment at week 8 of the trial protocol and were followed-up for an additional 4 weeks.[9] Among patients who continued sacubitril-valsartan for an additional 4 weeks, a further 17.2% reduction in NT-proBNP was observed; for patients who switched from enalapril to sacubitril-valsartan at week 8, a more significant 37.4% decline in NT-proBNP was seen over the following 4 weeks. Patients who started ARNI therapy in-hospital had a lower incidence of subsequent HHF or CV mortality through the entire 12-week trial period compared with patients who converted to ARNI after the first 8 weeks (13.0% vs 18.1%; P 1⁄4 0.03). A recent additional analysis has shown that the efficacy and safety of sacubitril-valsartan is generally similar across various dose levels,[10] supporting the rationale for in hospital initiation and continued post hospitalization use of sacubitril-valsartan broadly, including patients who might not tolerate early up-titration to target dose. Another recent analysis has shown the cost-effectiveness of this approach.[11]
Practical Tip
In patients suitable for switching to an ARNI, an ACEI can be discontinued at the time of hospital admission enabling ARNI prescription at 36 hours after admission. A 36 hour wash-out period is not necessary for those receiving ARB therapy at the time of hospitalization.
Recommendation
3. We recommend that an ARNI be used in place of an ACEI or ARB, in patients with HFrEF, who remain symptomatic despite treatment with appropriate doses of GDMT to decrease CV death, HF hospitalizations, and symptoms (Strong Recommendation; High-Quality Evidence).
4. We recommend that patients admitted to hospital for acute decompensated HF with HFrEF should be switched to an ARNI, from an ACEI or ARB, when stabilized and before hospital discharge (Strong Recommendation; Moderate-Quality Evidence).
5. We suggest that patients admitted to hospital with a new diagnosis of HFrEF should be treated with ARNI as first-line therapy, as an alternative to either an ACEI or ARB (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
These recommendation place high value on evidence that supports the safety and efficacy of initiating ARNI therapy in hospitalized patients with or without previous RASi exposure.
Practical Tip
In hospitalized and ambulatory patients with HF, without previous exposure to either an ACEI or ARB, an ARNI should be considered as first-line therapy when BP and renal function/potassium levels permit. Because a washout period is needed with ACEIs, initial therapy with this class in a hospitalized patient with HFrEF will delay the initiation of ARNI treatment.
ARNI might reduce diuretic requirements and diuretic dosing should be carefully evaluated when starting ARNI therapy.
Drug tolerability, side effects, and laboratory monitoring of ARNIs is similar to that of ACEIs or ARBs.
Appropriate clinical and laboratory follow-up (renal function and electrolytes) is essential after discharge to monitor for adverse events.
Currently, sacubitril-valsartan is the only available ARNI in Canada. Initial dosing and titration schedule should be individualized (Table 2).
ACEIs and ARBs
The benefits of GDMT for patients with HFrEF, including ACEIs and ARBs, are drawn from large randomized controlled trials of ambulatory patients. Previous guideline recommendations for ACEI/ARB therapy in patients with HFrEF reflect this evidence.[12] In contrast, recommendations regarding the role of RASi in the management of acute HF is largely consensus-based, with no good-quality evidence to support treatment recommendations in the hospitalized setting.[12] Practically, an HHF event represents an opportunity to optimize and/or reevaluate therapy including switch from an ACEI/ARB to an ARNI in eligible patients with HFrEF to improve postdischarge patient outcomes, as discussed in the previous section.
ACEI/ARB initiation and continuation during HF hospitalization. ACEIs and ARBs do not have a clear role in the early management of acute or worsening HF, because there are no robust randomized controlled trial data regarding in hospital ACEI/ARB initiation. Observational data from the Get With The Guidelines-HF Registry showed that among 16,052 patients, those who started ACEI/ARB treatment before discharge had lower mortality and readmission rates up to 1 year.[13] Nevertheless, a significant number of patients hospitalized for HFrEF have worsening hemodynamics and/or worsening renal function, which might lead to reluctance with initiating or continuing hemodynamically active therapies.[14]–[16] One analysis showed that ACEI/ARB medications were reduced or discontinued because of acute kidney injury (57%), hypotension (23%), and hyperkalemia (10%); serum creatinine and systolic at admission were significant independent predictors of in-hospital dose reduction or discontinuation.[17] Although renal dysfunction was noted as the most common cause for reduction of ACEI/ARB therapy, 24% of patients had no significant in-hospital rise in creatinine level, and medication changes were made in anticipation of deteriorating renal function rather than documented change in renal function.[17]
A matched-cohort analysis of Medicare beneficiaries hospitalized for HF between 1998 and 2001 showed that patients who initiated ACEI/ARB treatment had lower 30-day readmission rates (18% vs 24%) and all-cause mortality (7% vs 14%) compared with those for whom ACEI/ARB treatment was discontinued.[18]
ACEIs/ARBs after acute myocardial infarction. It is well established that ACEIs should be administered to patients with impaired LVEF (≤ 40%) or those who have experienced HF in the early phase post myocardial infarction (MI).[19]–[21] A systematic review[22] of 4 trials of early ACEI initiation (0-36 hours) post ST-elevation MI including more than 98,000 patients, showed a 7% relative reduction in 30-day mortality compared with placebo. Importantly, 40% of the survival benefit was seen after the first day of treatment, underscoring the value of initiating ACEI treatment early in hemodynamically stable patients.
ARBs as an alternative to ACEIs, in the context of ST-elevation MI, have been evaluated in 2 clinical trials. In the Optimal Trial in Myocardial Infarction With the Angiotensin II Antagonist Losartan (OPTIMAAL)[23] trial, losartan failed to show either superiority or noninferiority compared with captopril for the primary end point at the 2.7-year follow-up (18% vs 16%). Conversely, in the Valsartan in Acute Myocardial Infarction (VALIANT) trial,[19] 14,703 patients with acute MI (0.5 and 10 days) and HF or evidence of left ventricular systolic dysfunction ≤ 40% were randomly assigned to valsartan alone, full-dose captopril, or both (80 mg twice daily and 50 mg 3 times daily). The primary end point of all-cause mortality was similar in the 3 groups (valsartan 19.9%, captopril 19.5%, both 19.3%), but discontinuations were more frequently seen in patients who received captopril. Therefore, valsartan, at the dosages used in the trial, represents an alternative to ACEIs.
Practical Tip
ACEI intolerance describes a patient who is unable to tolerate ACEI therapy secondary to a bothersome cough (approximately 10%) or those who experience angioedema (< 1%). ARB therapy is a reasonable alternative in both of these cases, however, caution should be used in patients who develop angioedema while receiving ACEI therapy because there have been case reports of patients who subsequently develop angioedema with ARB therapy. There is no significant difference in rates of hypotension, hyperkalemia, or renal dysfunction between ACEIs and ARBs to warrant substitution.
Recommendation
6. We recommend an ACEI or ARB in those with ACEI intolerance, in patients with acute MI with HF or an LVEF < 40% post-MI to be used as soon as safely possible post-MI (Strong Recommendation; High-Quality Evidence).
Practical Tip
An increase in serum creatinine or decrease in estimated glomerular filtration rate (eGFR) of up to 30% in the absence of oliguria is not unexpected when an ACEI or ARB is introduced; if the increase stabilizes at 30%, there is no immediate need to decrease the drug dose but closer long-term monitoring might be required.
BP might fall when an ACEI or ARB is introduced, especially if introduced at a high dose or in combination with diuretic therapy. Check BP with the patient supine and standing to detect whether hypotension is present, which might suggest that a slower up-titration is warranted.
Caution is warranted in patients with marginal BP; although low-dose captopril is sometimes used to initiate an ACEI in hemodynamically tenuous patients this approach has never been tested in randomized controlled trials.
Longer-acting ACEIs such as perindopril or ramipril might be associated with less hypotension in patients with chronic HF, particularly in older patients.
β-Blockers
Since the 2017 comprehensive update of the CCS guidelines for the management of HF, no large randomized clinical trials of β-blockers in patients with HFrEF have been published. Previous landmark trials of carvedilol,[24],[25] sustained release metoprolol succinate,[26] and bisoprolol[27] have shown unequivocal reductions in mortality and hospitalization, and improvement in HF symptoms among patients with HFrEF and New York Heart Association (NYHA) functional class II-IV symptoms at baseline. In a meta-analysis of more than 10,000 patients, β-blockers prevented 3.8 deaths and were associated with 4 fewer hospitalizations per 100 patients in the first year of treatment.[28]
For patients admitted to hospital with worsening HF, β-blocker initiation, before discharge in stabilized patients, has been associated with improved short- and intermediate-term outcomes[29],[30] without intolerance or extended length of hospital stay. Available evidence also strongly suggests that patients with HFrEF receiving β-blockers at the time of admission for acute HF have higher rates of death and recurrent HHF when β-blockers are not resumed before discharge.[31]–[34]
A recent meta-analysis of 5 observational studies and 1 randomized trial confirmed this association; β-blocker withdrawal in the setting of HHF increased the risk of in-hospital mortality (RR, 3.72 [95% CI 1.51-9.14]), mortality at 60-180 days (RR, 1.78; [95% CI 1.13-2.79]), and combined short term rehospitalization or mortality (RR, 1.84; [95% CI 1.08-3.1]).[35] The totality of available evidence suggests that β-blockers should be continued or reinitiated before discharge in those with HFrEF who are hospitalized for worsening HF, whenever clinically feasible.
In addition to including β-blockers as part of standard medical HFrEF therapy, the following recommendations on β-blocker use in HFrEF have remained unchanged from the 2017 comprehensive update of the CCS guidelines for the management of HF.
Recommendation
7. We recommend that β-blockers be initiated as soon as possible after the diagnosis of HF, including during the index hospitalization, provided that the patient is hemodynamically stable. Clinicians should not wait until hospital discharge to start β-blocker treatment in stabilized patients (Strong Recommendation; High-Quality Evidence).
8. We recommend patients with NYHA class IV symptoms be stabilized before initiation of β-blocker treatment (Strong Recommendation; High-Quality Evidence).
9. We recommend that β-blockers be initiated in all patients with an LVEF < 40% with previous MI (Strong Recommendation; Moderate-Quality Evidence).
Practical Tip
Objective improvement in cardiac function might not be apparent for 6-12 months after β-blocker initiation. The absence of LVEF recovery is not justification to stop treatment.
Treatment of patients with NYHA class I or II symptoms can be safely initiated and titrated with a β-blocker by nonspecialist physicians.
Patients with NYHA class III or IV symptoms should have β-blocker therapy initiated by a specialist experienced in HF management and titrated in the setting of close follow-up, such as can be provided in a specialized clinic, if available.
β-Blockers should be started at low doses and increased slowly (eg, double the dose every 2-4 weeks). Transient fluid retention might occur with initiation or up-titration of β-blockers and might require assessment of diuretic dosage (eg, might consider deferring dosage reduction).
If concomitant reactive airways disease is present, consider using more selective β-1 blockade (eg, bisoprolol).
If atrioventricular (AV) block is present, consider decreasing other AV node-blocking drugs, such as digoxin or amiodarone (when appropriate). The type and severity of AV block and the patient’s history of arrhythmia will help guide the most appropriate treatment modifications.
MRAs
MRA use in patients with HFrEF. Despite access to MRA therapy for the treatment of HF, and despite established guideline recommendations to initiate MRAs as part of standard therapy (along with RASi and β-blocker medications), there remains uncertainty or reluctance for widespread use. A report of the recent US CHAMP-HF registry[36] showed that MRA was used in only 33.4% of patients with HFrEF without documented contraindication. On the basis of data from the Randomized Aldactone Evaluation Study (RALES),[37] the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS),[38] and the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF),[39] there are 3 clinical scenarios in which mineralocorticoid receptor antagonism in the absence of significant renal dysfunction or hyperkalemia are supported by randomized control trial evidence: (1) LVEF ≤ 35% and NYHA class III-IV symptoms; (2) post MI with signs and symptoms of acute HF and LVEF ≤ 40%, or post MI with diabetes and LVEF ≤ 40% (regardless of HF symptoms); and (3) LVEF ≤ 30% (or if LVEF 31%-35% with QRS > 130 ms), NYHA class II symptoms, and another high risk feature (eg, age > 55 years, HHF within the previous 6 months, or elevated natriuretic peptide levels).
A more generalized role for MRAs in HF management is further supported by contemporary trials that have shown a consistent benefit of newer therapies for which background treatment with MRAs has been > 50% among patients enrolled.[40],[41] Moreover, in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial HHF reduction was observed in patients with HF and LVEF ≥ 45% despite trial challenges in the population recruited,[42],[43] which might lessen the reluctance to treat HF patients on the basis of reduced ejection fraction alone.
Randomized controlled trial data regarding in-hospital initiation of MRA therapy among patients with HFrEF is limited to the EPHESUS trial. However, patients with worsening HF are often admitted to hospital, creating opportunity for improving HF therapies before discharge. In the PIONEER-HF study it was noted that in patients admitted with acute decompensated HF and reduced ejection fraction, 65% had a history of HF but only 10% were receiving an MRA at the time of admission.[8]
Patients with HF have multiple comorbidities adding complexity to their care. In-patient care for any one of these medical concerns is an opportunity to enhance HF therapy. In contrast, medications are often interrupted during acute medical illness and reintroduction at maximum tolerated doses before discharge is encouraged.
In addition to including MRAs as part of standard medical HFrEF therapy, the following recommendation has been updated.
Recommendation
10. We recommend MRA treatment for patients with acute MI and LVEF 40%, and HF symptoms or diabetes, to reduce mortality, CV mortality, and hospitalization for CV events (Strong Recommendation; High-Quality Evidence).
Practical Tip
MRAs recommended for patients with HFrEF include spironolactone and eplerenone.
MRAs should generally be avoided when eGFR is < 30 mL/min/1.73 m2.
MRAs can increase serum potassium, especially during an acute dehydrating illness in which renal dysfunction can worsen. Monitoring of serum creatinine and potassium should be repeated within 1 week of initiation or dose change.
Temporary reduction or interruption of MRA therapy might be necessary when potassium levels are moderately (5.6-5.9 mmol/L) or severely (> 5.9 mmol/L) elevated, with a return to maximum tolerated dose when other modifiable factors are corrected and potassium levels are ≤ 5.0 mmol/L.
MRAs, when used for HF, have very little effect on BP.
SGLT2 inhibitors
When to start SGLT2 inhibitor treatment in patients with HFrEF. The benefits of SGLT2 inhibitors in patients with established HFrEF have been shown in 2 large clinical trials and 1 meta-analysis, with consistency of benefit regardless of diabetes status.[40],[41],[44] These agents should be considered as standard or foundational therapy in patients with HFrEF (Fig. 1).
The results of the Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction (DAPA-HF) trial were described in the previous CCS HF guideline update.[2] Over a median 18-month follow-up of 4744 patients with HFrEF, treatment with dapagliflozin significantly reduced the composite primary end point of time to first worsening of HF or death from CV causes (hazard ratio [HR], 0.74 [95% CI 0.65-0.85]; P < 0.001), as well as HHF (HR, 0.70 [95% CI 0.59 – 0.83]) and CV death (HR, 0.82 [95% CI 0.69 – 0.98]). Importantly, 55% of patients in this trial did not have diabetes at baseline, and the effect of dapagliflozin was similar at any hemoglobin A1c level.[40] Ancillary studies have shown that benefits accrued as early as 30 days after treatment initiation.[45] Other notable substudy findings were that diuretic dose was not modified during the trial for most patients,[46] quality of life was improved,[47] and BP was reduced by an average of approximately 2 mm Hg.[48] Importantly, baseline kidney function did not modify the effect of dapagliflozin on outcomes and treatment was associated with a slower eGFR decline compared with placebo in diabetic and nondiabetic cohorts.[49]
The results of the recently published EMPEROR-Reduced trial,[41] in which empagliflozin 10 mg daily was compared with placebo in patients with symptomatic HFrEF, were concordant with those of DAPA-HF. Participants included those with an LVEF < 40% and elevated NT-proBNP levels that varied according to LVEF and atrial fibrillation status. Enrollment could occur with an eGFR as low as 20 mL/min/1.73 m2. During a median follow-up of 16 months, the primary outcome of CV death or HHF occurred in 19.4% of participants in the empagliflozin group and in 24.7% of the placebo group (HR, 0.75 [95% CI 0.65-0.86]; P < 0.001); this benefit was comparable for patients with and without diabetes. The total number of HHF was lower in the empagliflozin group (HR, 0.70 [95% CI 0.58-0.85]; P < 0.001), as was the annual rate of decline in eGFR (0.55 vs 2.28 mL/min/1.73 m2 per year; P < 0.001).
The use of background pharmacological therapy for HFrEF was excellent in both trials. Of particular note, sacubitril-valsartan served as a RASi among approximately 11% of patients in DAPA-HF and in approximately 19% in EMPEROR-Reduced at enrollment. Cardiac resynchronization therapy (CRT) was used in 7.5% of participants in DAPA-HF and in 12% of those in EMPEROR-Reduced, whereas implantable cardioverter defibrillators (ICDs), with or without CRT, were used in 26% and 31%, respectively. There were no treatment interactions between SGLT2 inhibitor and the baseline therapies used. SGLT2 inhibitor treatment was safe with no excess in hypovolemia, hypoglycemia, or renal side effects compared with placebo.
Taken together, as shown in a meta-analysis by Zannad and colleagues, the results of these 2 landmark trials show that SGLT2 inhibitor reduces morbidity and mortality in patients with symptomatic HFrEF, whether type 2 diabetes is present or not.[44]
The recently published Dapagliflozin in Patients With Chronic Kidney Disease (DAPA-CKD) trial[50] showed that dapagliflozin, when used in addition to standard therapy, also prevents renal and CV outcomes in patient with established chronic kidney disease. Among 4304 participants, with or without type 2 diabetes, with an eGFR between 25 and 75 mL/min/1.73 m2 and proteinuria (a urinary albumin-to-creatinine ratio of 22.6-565.6 mg/mmol) who were randomly assigned to dapagliflozin 10 mg daily or placebo, the primary composite outcome of a sustained decline in eGFR of at least 50%, end-stage kidney disease, or death from renal or CV causes was reduced by 44% (HR, 0.56 [95% CI 0.45-0.68]; P < 0.001). The hazard ratio for the composite of death from CV causes or HHF was 0.71 ([95% CI 0.55-0.92]; P 1⁄4 0.009). All-cause mortality was also significantly reduced (HR, 0.69; [95% CI 0.53-0.88]; P 1⁄4 0.004) and the safety profile of dapagliflozin was confirmed in this group.
Recommendation
11. We recommend an SGLT2 inhibitor, such as dapagliflozin or empagliflozin, be used in patients with HFrEF, with or without concomitant type 2 diabetes, to improve symptoms and quality of life and to reduce the risk of HF hospitalization and/or CV mortality (Strong Recommendation; High-Quality Evidence).
12. We recommend an SGLT2 inhibitor, such as empagliflozin, canagliflozin, or dapagliflozin be used for treatment of patients with type 2 diabetes and atherosclerotic CV disease to reduce the risk of HF hospitalization and death (Strong Recommendation; High-Quality Evidence).
13. We recommend an SGLT2 inhibitor, such as dapagliflozin, be used in patients with type 2 diabetes who are older than 50 years with additional risk factors for atherosclerotic CV disease to reduce the risk of HF hospitalization (Strong Recommendation; High-Quality Evidence).
14. We recommend SGLT2 inhibitors such as canagliflozin or dapagliflozin be used in patients with albuminuric renal disease, with or without type 2 diabetes, to reduce the risk of HF hospitalization and progression of renal disease (Strong Recommendation; High-Quality Evidence).
Values and Preferences
These recommendations place weight on the results from large randomized, placebo- controlled trials that consistently showed a benefit of SGLT2 inhibitor treatment on HF prevention and treatment among patients with and without type 2 diabetes.
Practical Tip
In EMPEROR-Reduced and DAPA-HF, SGLT2 inhibitor treatment was initiated in addition to maximally tolerated GDMT. However, recognizing the significant residual risk of patients with HFrEF despite GDMT and the benefits associated with dapagliflozin and empagliflozin, it would be reasonable to start this class of therapy early in the disease course for eligible patients.
EMPEROR-Reduced excluded patients with an eGFR < 20 mL/min/1.73 m2 and DAPA-HF excluded patients with an eGFR < 30 mL/min/1.73 m2. Data supporting the use of these agents in patients with HFrEF and eGFR < 30 mL/min/1.73 m2 are very limited.
The Canadian Heart Failure Society (CHFS) has published “Practical Approach to SGLT2 Inhibitors for Treatment of Cardiovascular Disease,” which includes contraindications, cautions, drug initiation, special considerations, and sick day management tips.[51] Additional Practical Tips related to SGLT2 inhibitor prescription from the previous 2020 HF guideline update2 remain relevant and are included as follows:
SGLT2 inhibitors are currently contraindicated for patients with type 1 diabetes.
The most common adverse effect of this class of medications are genital mycotic infections (GMIs). Women (10%-15% risk), those with previous GMIs, and uncircumcised men are at highest risk. Typically, GMIs can be managed with antifungal drugs and do not require discontinuation of therapy.
SGLT2 inhibitor use might result in temporary reduction of eGFR up to 15%, which generally resolves within 1-3 months. SGLT2 inhibitors have also been associated with acute kidney injury and increased monitoring is warranted in those at risk.
SGLT2 inhibitors rarely cause hypoglycemia in the absence of concomitant insulin and/or secretagogue therapy. Background therapies might need to be adjusted to prevent hypoglycemia.
SGLT2 inhibitors should be held in the setting of concomitant dehydrating illness as part of “Sick Day” management. Patients should be educated on “Sick Day” management.
These agents have been associated with diabetic ketoacidosis (incidence 0.1%). Patients might present with normal or only modestly elevated blood glucose level (< 14 mmol/L). On rare occasions, SGLT2 inhibitors might be associated with normal anion gap acidosis, which is best detected with measurement of serum ketones. Nonspecific symptoms associated with diabetic ketoacidosis include: shortness of breath, nausea, vomiting, abdominal pain, confusion, anorexia, excessive thirst, and lethargy.
Careful attention to volume status is required when SGLT2 inhibitors, ARNIs, and loop diuretics are used in combination because of their concomitant effects to promote diuresis.
References
4. Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol 2019;73:2365-83.
5. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993-1004.
6. Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail 2019;21:998-1007.
7. Pascual-Figal D, Wachter R, Senni M, et al. NT-proBNP response to sacubitril/valsartan in hospitalized heart failure patients with reduced ejection fraction: TRANSITION study. JACC Heart Fail 2020;8:822-33.
8. Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med 2019;380:539-48.
9. DeVore AD, Braunwald E, Morrow DA, et al. Initiation of angiotensin-neprilysin inhibition after acute decompensated heart failure: secondary analysis of the open-label extension of the PIONEER-HF trial. JAMA Cardiol 2020;5:202-7.
10. Berg DD, Braunwald E, DeVore AD, et al. Efficacy and safety of sacubitril/valsartan by dose level achieved in the PIONEER-HF trial. JACC Heart Fail 2020;8:834-43.
11. Grant ADM, Chew DS, Howlett JG, Miller RJH. Cost-effectivness of earlier transition to angiotensin receptor neprilysin inhibitor in patients with heart failure and reduced ejection fraction. CJC Open 2020;2: 447-53.
12. McKelvie RS, Moe GW, Ezekowitz JA, et al. The 2012 Canadian Cardiovascular Society heart failure management guidelines update: focus on acute and chronic heart failure. Can J Cardiol 2013;29:168-81.
13. Gilstrap LG, Fonarow GC, Desai AS, et al. Initiation, continuation, or withdrawal of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and outcomes in patients hospitalized with heart failure with reduced ejection fraction. J Am Heart Assoc 2017;6:e004675.
14. Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol 2009;53:557-73.
15. Greene SJ, Fonarow GC, Vaduganathan M, et al. The vulnerable phase after hospitalization for heart failure. Nat Rev Cardiol 2015;12:220-9.
16. Vaduganathan M, Butler J, Pitt B, Gheorghiade M. Contemporary drug development in heart failure: call for hemodynamically neutral therapies. Circ Heart Fail 2015;8:826-31.
17. Kane JA, Kim JK, Haidry SA, Salciccioli L, Lazar J. Discontinuation/dose reduction of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers during acute decompensated heart failure in African-American patients with reduced left-ventricular ejection fraction. Cardiology 2017;137:121-5.
18. Sanam K, Bhatia V, Bajaj NS, et al. Renin-angiotensin system inhibition and lower 30-day all-cause readmission in Medicare beneficiaries with heart failure. Am J Med 2016;129:1067-73.
19. Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 2003;349:1893-906.
20. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) study investigators. Lancet 1993;342:821-8.
21. Kober L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med 1995;333:1670-6.
22. Indications for ACE inhibitors in the early treatment of acute myocardial infarction: systematic overview of individual data from 100,000 patients in randomized trials. ACE Inhibitor Myocardial Infarction Collaborative Group. Circulation 1998;97:2202-12.
23. Dickstein K, Kjekshus J; OPTIMAAL Trial Steering Committee, for the OPTIMAAL Study Group. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet 2002;360:752-60.
24. Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 1996;334:1349-55.
25. Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-8.
26. Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the Metoprolol CR/XL Randomized Intervention Trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000;283:1295-302.
27. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13.
28. Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med 2001;134:550-60.
29. Gattis WA, O’Connor CM, Gallup DS, et al. Predischarge initiation of carvedilol in patients hospitalized for decompensated heart failure: results of the Initiation Management Predischarge: Process for Assessment of Carvedilol Therapy in Heart Failure (IMPACT-HF) trial. J Am Coll Cardiol 2004;43:1534-41.
30. Bohm M, Link A, Cai D, et al. Beneficial association of beta-blocker therapy on recovery from severe acute heart failure treatment: data from the Survival of Patients With Acute Heart Failure in Need of Intravenous Inotropic Support trial. Crit Care Med 2011;39:940-4.
31. Jondeau G, Neuder Y, Eicher JC, et al. B-CONVINCED: Beta-blocker CONtinuation Vs. INterruption in patients with Congestive heart failure hospitalizED for a decompensation episode. Eur Heart J 2009;30:2186-92.
32. Fonarow GC, Abraham WT, Albert NM, et al. Influence of beta-blocker continuation or withdrawal on outcomes in patients hospitalized with heart failure: findings from the OPTIMIZE-HF program. J Am Coll Cardiol 2008;52:190-9.
33. Butler J, Young JB, Abraham WT, et al. Beta-blocker use and outcomes among hospitalized heart failure patients. J Am Coll Cardiol 2006;47:2462-9.
34. Gattis WA, O’Connor CM, Leimberger JD, et al. Clinical outcomes in patients on beta-blocker therapy admitted with worsening chronic heart failure. Am J Cardiol 2003;91:169-74.
35. Prins KW, Neill JM, Tyler JO, Eckman PM, Duval S. Effects of beta-blocker withdrawal in acute decompensated heart failure: a systematic review and meta-analysis. JACC Heart Fail 2015;3:647-53.
36. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol 2018;72:351-66.
37. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999;341:709-17.
38. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003;348:1309-21.
39. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011;364:11-21.
40. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995-2008.
41. Packer M, Anker SD, Butler J, et al. Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-Reduced trial. Circulation 2021;413:326-36.
42. Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014;370:1383-92.
43. Solomon SD, Claggett B, Lewis EF, et al. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J 2016;37:455-62.
44. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inibitors in patients with heart failure and reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:819-29.
45. Martinez FA, Serenelli M, Nicolau JC, et al. Efficacy and saftey of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA-HF. Circulation 2020;141:100-11.
46. Jackson AM, Dewan P, Anand IS, et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Circulation 2020;142:1040-54.
47. Kosiborod MN, Jhund PS, Docherty KF, et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results fro the DAPA-HF trial. Circulation 2020;141:90-9.
48. Serenelli M, Bohm M, Inzucchi SE, et al. Effect of dapagliflozin according to baseline systolic blood pressure in the dapagliflozin and prevention of adverse outcomes in heart failure trial (DAPA-HF). Eur Heart J 2020;41:3402-18.
49. Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure and reduced ejection fraction: results of DAPA-HF. Circulation 2021;143:298-309.
50. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436-46.
