4. Practical Considerations for Integrating GLP-1RA and SGLT2i Into Practice
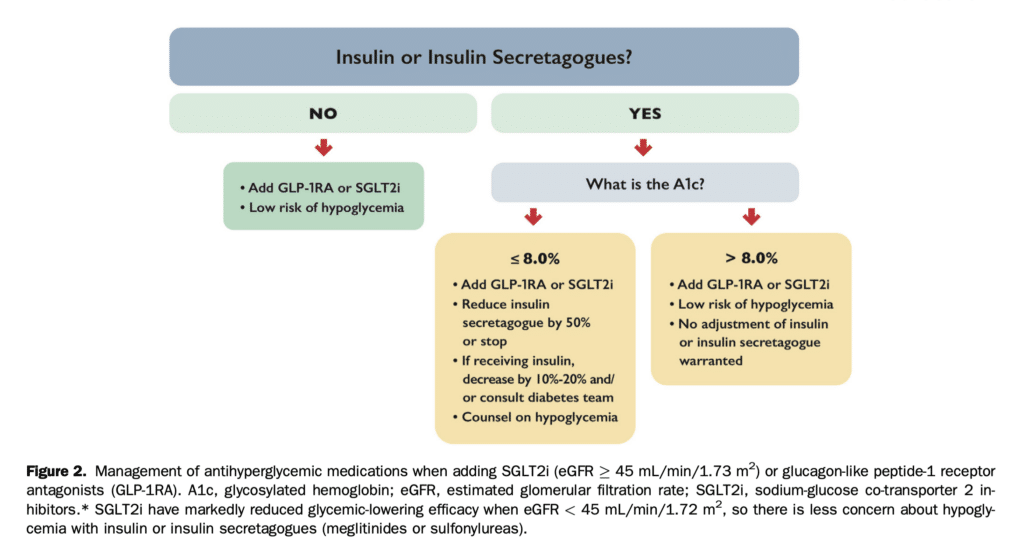
When using these classes for cardiorenal benefit, one must consider potential side effects and advise on strategies to minimize them (Table 4 and Fig. 2).[3-5],[60-65]
The most common side effects of GLP-1RA are gastrointestinal (nausea, vomiting, diarrhea). These side effects are most prominent at initiation of treatment and usually improve over time. Mitigation strategies are shown in Table 4. Note that a dipeptidyl peptidase 4 (DPP-4) inhibitor can be discontinued when adding GLP-1RA because they are both incretin-based therapies and the DPP-4 inhibitor is redundant in that situation. In the SUSTAIN 6 trial of subcutaneous once weekly semaglutide, more retinopathy events occurred in the semaglutide group. However, this increase appears to be due to the rapid and robust glucose-lowering in people with marked hyperglycemia and preexisting retinopathy – phenomenon previously observed in studies of insulin. In those patients, glucose-lowering is still desired but simultaneous regular examinations by an eye care professional is critical so that any changes can be addressed in a timely fashion. The long-term benefits of glucose control on the eyes far outweigh any acute risk.
The most common adverse effect of SGLT2i is genital mycotic infections (GMIs). Women (10%-15% risk), those with previous GMI, and uncircumcised men are at highest risk. GMI risk can be reduced with appropriate genital hy- giene strategies and when they occur, can typically be managed with antifungal drugs and do not require discon- tinuation of therapy.
The risk of hypoglycemia is an important consideration if patients are using insulin secretagogues (meglitinides and sulfonylureas) and/or insulin (Fig. 2). The risk of hypogly- cemia is greater if the eGFR is > 45 mL/min/m2 or the A1c is close to target in which case a reduced dose of insulin secre- tagogues and/or insulin should be considered and additional self-blood glucose monitoring and counselling around hypoglycemia symptoms and treatment are recommended. The risk of hypoglycemia is lower if A1c is > 8%. If SGLT2i are used in those with eGFR < 45 mL/min/1.73 m2 the risk is lower because the glycemic-lowering effect of SGLT2i is minimal. In contrast, for GLP-1RA, the potential for glucose-lowering is present across the eGFR spectrum. To decide if adjustment of existing insulin secretagogues and/or insulin is needed, consider the current A1c. If A1c is > 8%, then the addition of these agents is less likely to cause hypoglycemia but the patient should be counselled about the potential for hypoglycemia. However, if the A1c is <= 8%, then a reduction in the dose or discontinuation of the insulin secretagogue is warranted to avoid hypoglycemia. In the case of insulin, dose reduction by 10%-20% or more might be required to avoid hypoglycemia. Communication with the patient’s diabetes team is critical when any such changes to therapy are being considered, especially for SGLT2i, as aggressive reduction of insulin is a risk factor for DKA.
SGLT2i have been associated with DKA (incidence 0.1%) among patients with diabetes. Patients with SGLT2i-associated DKA might present with normal or only modestly elevated blood glucose level (< 14 mmol/L). Inadequate insulin remains the cause of DKA and therefore, mitigation strategies shown in Table 4 can reduce the risk. Nonspecific symptoms associated with DKA include: shortness of breath, nausea, vomiting, abdominal pain, confusion, anorexia, excessive thirst, and lethargy. Patients without diabetes are not at risk of DKA when these agents are used.[64]
SGLT2i use might result in a temporary decrease in eGFR of up to 15%-25%, which generally resolves in 1-3 months and is not usually a sign of acute kidney injury. The decrease in eGFR is expected and results from a decrease in intra-glomerular pressure induced by these agents, conceptually similar to what is seen with ACEi and ARB. In fact, trial evidence suggests that there is no increase or even a reduction in acute kidney injury.[61] Accordingly, this eGFR reduction should not result in premature discontinuation of SGLT2i, which favourably modifies kidney outcomes. Despite this reassurance, attention to volume status is always required, especially when SGLT2i, ARNIs, and loop diuretics are used in combination because of their additive effects to promote diuresis. SGLT2i should be stopped temporarily in the setting of concomitant dehydrating illness as part of “sick day” management.[62] Caution is warranted in patients with very low and variable BP or when kidney function is already extremely compromised. Currently, canagliflozin and dapagliflozin are contraindicated in patients undergoing dialysis and empagliflozin is contraindicated in patients with an eGFR < 20 mL/ min/1.73 m2. As indicated previously, referral to a specialist with expertise in CKD should be considered in the following situations: progressive loss of kidney function, urine UACR persistently > 60 mg/mmol, or progressive increase in UACR despite appropriate therapy, eGFR < 30 mL/min/1.73 m2, inability to continue kidney-protective therapies because of adverse effects, such as hyperkalemia or a > 30% increase in serum creatinine within 3 months of starting SGLT2i, ACEi, or ARB, inability to achieve target BP, or signs/symptoms of another underlying kidney disease, such as glomerulonephritis.
The increased risk of amputation seen in the large, long-term Canagliflozin Cardiovascular Assessment Study (CANVAS) trial for canagliflozin, and select observational studies, merits further research but overall, there is currently no consistent evidence of SGLT2i exposure and increased risk of amputation.[65]

References
3. Lipscombe L, Butalia S, Dasgupta K, et al. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can J Diabetes 2020;44:575-91.
4. Senior PA, Houlden RL, Kim J, et al. Pharmacologic glycemic man- agement of type 2 diabetes in adults: 2020 update – the user’s guide. Can J Diabetes 2020;44:592-6.
5. O’Meara E, McDonald M, Chan M, et al. CCS/CHFS heart failure guidelines: clinical trial update on functional mitral regurgitation, SGLT2 inhibitors, ARNI in HFpEF, and tafamidis in amyloidosis. Can J Cardiol 2020;36:159-69.
60. Lingvay I, Leiter LA. Use of GLP-1 RAs in cardiovascular disease prevention: a practical guide. Circulation 2018;137:2200-2.
61. Lin DS, Lee JK, Chen WJ. Clinical adverse events associated with sodium-glucose cotransporter 2 inhibitors: a meta-analysis involving 10 randomized clinical trials and 71 553 individuals. J Clin Endocrinol Metab 2021;106:2133-45.
62. Diabetes Canada Clinical Practice Guidelines Expert Committee. Diabetes Canada 2018 clinical practice guidelines for the prevention and management of diabetes in canada: sick-day medication list. Can J Diabetes 2018;42:S316.
63. Canadian Heart Failure Society. Practical approach to SGLT2 inhibitors for treatment of cardiovascular disease. Available at: https://heartfailure.ca/ sites/default/files/chfs_practical_approach_algorithm_sglt2i.pdf. Accessed November 8, 2021.
64. Goldenberg RM. Sodium-glucose cotransporter-2 inhibitors increase the risk of diabetic ketoacidosis in adults with type 2 diabetes: should we be concerned in people without diabetes? Can J Diabetes 2022;46:109.
65. Fralick M, Kim SC, Schneeweiss S, Everett BM, Glynn RJ, Patorno E. Risk of amputation with canagliflozin across categories of age and cardiovascular risk in three US nationwide databases: cohort study. BMJ 2020;370:m2812.
