2. Integration of SGLT2i in the Management of Patients With CKD
PICO 3: In patients with CKD what is the role of novel anti-hyperglycemic agents compared with placebo for reduction of the composite of kidney death, progression to dialysis, or reduction of eGFR?
An ongoing study is evaluating the role of GLP-1RA in patients with established CKD43 but completed trials pertain solely to SGLT2i. The first trial in the setting of CKD was the Canagliflozin and Renal Events in Diabetes With Established Nephropathy Clinical Evaluation (CREDENCE) trial undertaken in patients with T2D, eGFR 30 to < 90 mL/min/1.73 m2 and UACR 33.9-565 mg/mmol.[16] All patients were receiving baseline ACEi or ARB, and were assigned to treatment with canagliflozin at a dose of 100 mg daily or placebo. The trial was stopped early (with 4401 patients randomized and a median follow-up of 2.6 years) because of overwhelming benefit: the composite of end-stage kidney disease, doubling of serum creatinine, and kidney or CV death was reduced in subjects who received canagliflozin compared with placebo (43.2 vs 61.2 events per 1000 patient-years; P < 0.00001). Beneficial effects were noted irrespective of baseline A1c, including among patients with A1c between 6.5% and 7%. The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial[17] showed that dapagliflozin, used in addition to standard therapy, also reduced kidney and CV outcomes in patients with established CKD. In 4304 participants, with or without T2D, with an eGFR between 25 and 75 mL/min/1.73 m2 and albuminuria (a UACR of 22.6-565.6 mg/mmol) who were randomized to dapagliflozin 10 mg daily or placebo, the primary composite of a sustained decline in eGFR of at least 50%, end-stage kidney disease, or death from kidney or CV causes was reduced by 44% (HR, 0.56; 95% CI, 0.45-0.68; P < 0.001). The HR for the composite of death from CV causes or hospitalization for HF was 0.71 (95% CI, 0.55-0.92; P < 0.009). All-cause mortality was also significantly reduced (HR, 0.69; 95% CI, 0.53-0.88; P < .004) and the excellent safety profile of dapagliflozin was confirmed in this group. Not available at the time of data synthesis and publication of this guideline, the Empagliflozin Once Daily to Assess Cardio-renal Outcomes in Patients With Chronic Kidney Disease (EMPA-KIDNEY) study, undertaken in patients with established CKD, which compared empagliflozin 10 mg with placebo, was stopped early after achieving positive efficacy on the basis of the primary end point (a composite of kidney disease progression or cardiovascular death) thereby further supporting the role of SGLT2i for cardiorenal protection.[44]
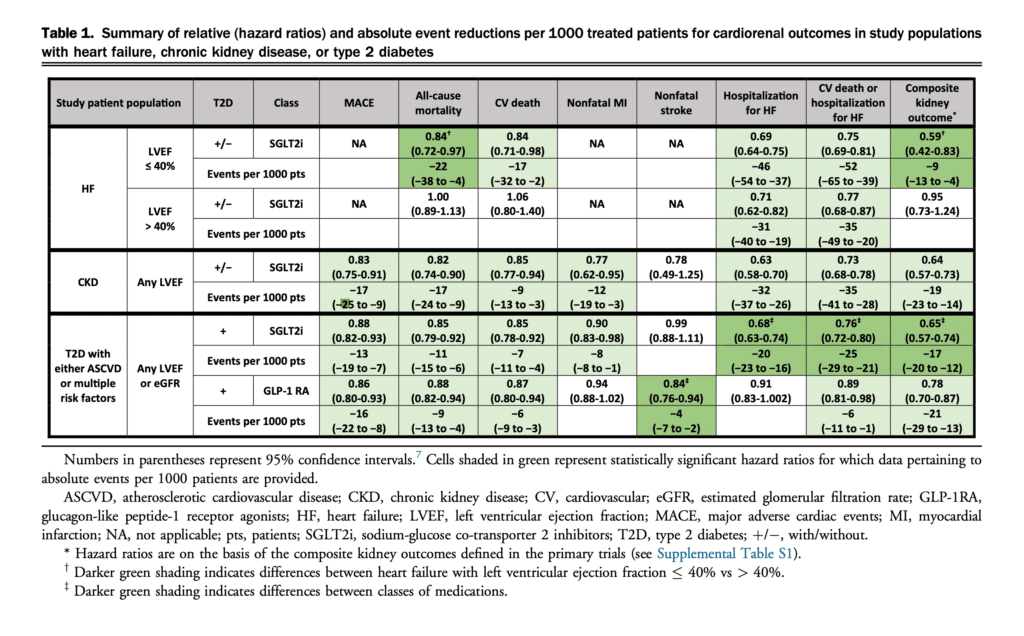
On the basis of our meta-analysis of the available data, Table 1 shows substantial benefit in all critical end points of interest except for reduction of nonfatal stroke.[7] The 36% reduction in the composite kidney outcome was also associated with all-cause (18%) and CV mortality (15%) reductions, a 23% reduction in nonfatal MI, and a 37% reduction in hospitalization for HF.[7]
RECOMMENDATION
3. In adults with CKD (UACR > 20 mg/mmol and eGFR >= 25 mL/min/1.73 m2), we recommend use of SGLT2i to reduce the composite of significant decline in eGFR, progression to end-stage kidney disease, or kidney death, all-cause and CV mortality, nonfatal MI, and hospitalization for HF (Strong recommendation, Moderate-Quality Evidence).
Practical tip
Referral to a specialist with expertise in CKD should be considered in the following situations: progressive loss of kidney function, urine UACR persistently > 60 mg/ mmol, or progressive rise in UACR despite appropriate therapy, eGFR < 30 mL/min/1.73 m2, inability to continue kidney-protective therapies because of adverse effects, such as hyperkalemia or a > 30% increase in serum creatinine within 3 months of starting SGLT2i, ACEi, or ARB, inability to achieve target BP, or signs/symptoms of another underlying kidney disease, such as glomerulonephritis.
General discussion
Because of the close inter-relationship of CKD and CV disease, recognizing these clinical entities and their prognostic effect is of great importance. A diagnosis of CKD is made in people with an eGFR < 60 mL/min/1.73 m2 and/or random UACR >= 2.0 mg/mmol on at least 2 of 3 samples over a 3-month period.6 Measuring eGFR and UACR on an annual basis (at a minimum) is recommended for patients with CV disease or multiple risk factors. The reviewed trials indicate cardiorenal benefits of SGLT2i in patients regardless of diabetes status. Among those living with CKD and T2D, it should be emphasized that reduction in the onset and progression of CKD can also be enhanced by attaining optimal A1c and BP goals. For the latter, incorporation of an ACEi or an ARB is warranted. Moreover, even when patients with T2D and CKD have achieved A1c goals, one of the SGLT2i should be included to reduce risks of CKD progression, HF, and MACE. In patients with T2D and CKD who have not achieved individualized glycemic targets despite use of metformin and SGLT2i, or who are unable to use those medications, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines6 recommend long-acting GLP-1RA. This is further supported by secondary analyses of some of the GLP-1RA trials in patients with T2D.[45] Although this guideline supports this recommendation, we have not made an explicit recommendation in this regard because no dedicated CKD trials using this class are currently complete. The available evidence from other trials is summarized in the accompanying de novo meta-analysis7 but the kidney outcomes definitions have varied among trials (Supplemental Table S1). Moreover, because of our focus on cardiorenal benefits, and considering that SGLT2i have been studied in clinical trials dedicated to patients with CKD, we believe that SGLT2i should be part of first-line treatment in patients with CKD and T2D and that the inclusion of drugs with proven cardiorenal benefits should be independent of whether the patient is taking metformin or not. It should be emphasized, however, that the A1c-lowering effect of SGLT2i is diminished in the presence of CKD, and is minor at eGFR 30-45 mL/min/1.73 m2 and absent at an eGFR of < 30 mL/min/ 1.73 m2.
References
6. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2020;98:S1-115.
7. Ali MU, Mancini GBJ, Fitzpatrick-Lewis D, et al. The effectiveness of sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists on cardiorenal outcomes: systematic review and meta-analysis. Can J Cardiol 2022;38:1201-10.
16. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295-306.
17. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436-46.
43. ClinicalTrials.gov. A research study to see how semaglutide works compared to placebo in people with type 2 diabetes and chronic kidney disease (FLOW). Available at: https://clinicaltrials.gov/ct2/show/ NCT03819153. Accessed November 29, 2021.
44. EMPA-Kidney Collaborative Group. Design, recruitment, and baseline characteristics of the EMPA-KIDNEY trial. Nephrol Dial Transplant 2022;37:1317-29.
45. Shaman AM, Bain SC, Bakris GL, et al. Effect of the glucagon-like peptide-1 receptor agonists semaglutide and liraglutide on kidney outcomes in patients with type 2 diabetes: a pooled analysis of SUSTAIN 6 and LEADER trials. Circulation 2022;145:575-85.
