1. SGLT2i for the Treatment of HF
PICO 1: In patients with HF and reduced ejection fraction (HFrEF; <= 40%) what is the role of SGLT2i and GLP-1RA compared with placebo for reduction of CV disease or hospitalization for HF?
Our systematic review did not identify any large randomized clinical trials of GLP-1RA for the management of HF. Accordingly, our discussion and recommendations are on the basis of evidence from clinical trials of SGLT2i. The results of the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) trial[18] were described in the previous CCS/ CHFS guideline update.[5] During a median 18-month follow-up of 4744 patients with HFrEF, treatment with dapagliflozin 10 mg daily significantly reduced the composite primary endpoint of time to first worsening of HF or death from CV causes (HR, 0.74; 95% confidence interval [CI], 0.65-0.85]; P < 0.001), as well as hospitalization for HF (HR, 0.70; 95% CI, 0.59-0.83) and CV death (HR, 0.82; 95% CI, 0.69-0.98). Importantly, 55% of patients did not have T2D, and the effect of dapagliflozin was similar at any A1c level.[18] Ancillary studies have shown that benefits were seen as early as 30 days after treatment initiation.[28] Additionally, diuretic dose was not modified during the trial for most patients,[29] quality of life was improved,[30] and blood pressure (BP) was reduced by an average of approximately 2 mm Hg.[31] Outcomes were not modified by baseline kidney function and dapagliflozin was associated with a slower eGFR decline compared with placebo in diabetes and nondiabetes cohorts.[32] The Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Reduced Ejection Fraction (EMPEROR-Reduced) trial,[21] which compared empagliflozin 10 mg daily with placebo in patients with symptomatic HFrEF, showed results concordant with the DAPA-HF study. Participants had an LVEF <= 40% and elevated N-terminal pro hormone brain natriuretic peptide levels that varied according to LVEF and atrial fibrillation status. Enrollment could occur with an eGFR as low as 20 mL/min/1.73 m2. During a median follow-up of 16 months, CV death or hospitalization for HF occurred in 19.4% of participants in the empagliflozin group and in 24.7% of the placebo group (HR, 0.75; 95% CI, 0.65- 0.86; P < 0.001) and the benefit was comparable in those with or without diabetes. The total number of hospitalizations for HF was lower in the empagliflozin group (HR, 0.70; 95% CI, 0.58- 0.85; P < 0.001), as was the annual rate of decline in eGFR (-0.55 vs -2.28 mL/min/1.73 m2 per year; P < 0.001). Use of background therapy for HFrEF was excellent in both trials.
Notably, sacubitril-valsartan served as a renin-angiotensin inhibitor in approximately 11% of patients in DAPA-HF and approximately 19% in EMPEROR-Reduced at baseline (concordant with clinical practice at the time of recruitment for these trials). Cardiac resynchronization therapy was used in 7.5% of patients in DAPA-HF and in 12% in EMPEROR-Reduced. Implantable cardioverter defibrillators, with or without cardiac resynchronization therapy, were used in 26% and 31%, respectively. No treatment interactions were noted among SGLT2i and these baseline therapies.[21] Treatment with SGLT2i showed no excess in hypovolemia, hypoglycemia, or renal side effects compared with placebo. A meta-analysis of the 2 trials shows that SGLT2i reduces morbidity and mortality in patients with symptomatic HFrEF, whether T2D is present or not.[33] The CCS/CHFS guideline was one of the first worldwide to endorse SGLT2i as foundational therapy for patients with HFrEF in concert with angiotensin receptor neprilysin inhibitor (ARNI), or angiotensin-converting enzyme inhibitor (ACEi)/ angiotensin receptor blocker (ARB), b-blocker, and mineralocorticoid receptor antagonist (MRA).[5]
Our systematic review and meta-analysis (Table 1) indicates that use of SGLT2i in patients with LVEF <= 40% is associated with a 16% reduction in all-cause mortality or CV mortality, a 31% reduction in hospitalization for HF, and a 41% reduction in the composite kidney outcome of significant decline in eGFR, progression to end-stage kidney disease, or death due to kidney disease.[7]
RECOMMENDATION
1. In adults with HF and LVEF <= 40%, we recommend use of SGLT2i to reduce all-cause and CV mortality, hospitalization for HF, and the composite endpoint of significant decline in eGFR, progression to end-stage kidney disease, or death due to kidney disease (Strong Recommendation; Moderate-Quality Evidence).
Practical tip
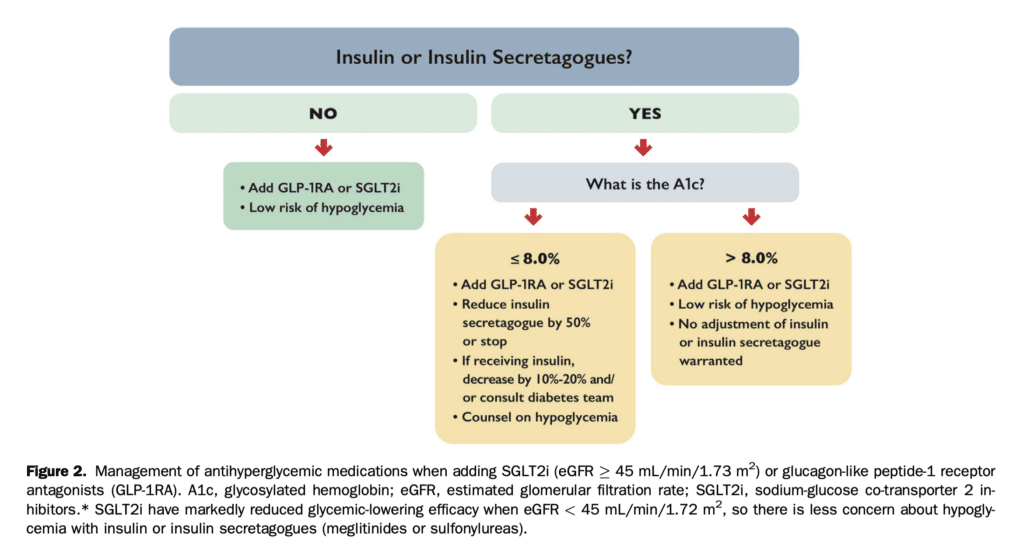
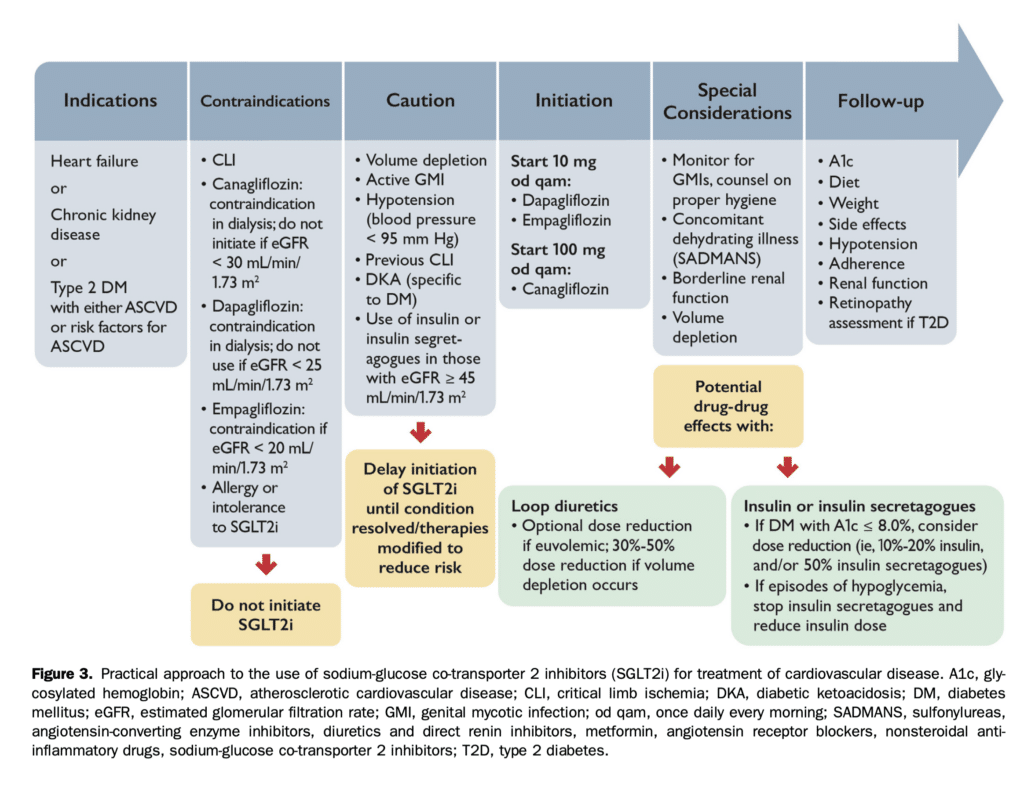
SGLT2i can be considered in stabilized HF patients. They are not indicated for the treatment of type 1 diabetes, or for patients receiving dialysis or with severely compromised renal function (eGFR < 20 mL/min/1.73 m2). Clinicians should refer to the appropriate guidelines for conditions such as symptomatic hyperglycemia, metabolically decompensated patients with T2D, as well as for acute renal failure. Consider temporary discontinuation of SGLT2i therapy in the context of acute events (see Figs. 2 and 3), and permanent discontinuation if eGFR remains < 20 mL/min/1.73 m2.
PICO 2: In patients with HF and preserved ejection fraction (HFpEF; > 40%) what is the role of novel antihyperglycemic agents compared with placebo for reduction of CV death or hospitalization for HF?
The Empagliflozin Outcome Trial in Patients With Chronic Heart Failure With Preserved Ejection Fraction (EMPEROR-Preserved)[22] trial was the first phase III randomized double-blind placebo-controlled trial to achieve its primary endpoint in patients with symptomatic HFpEF (> 40%). In this landmark trial the composite of CV death or HF hospitalization was significantly reduced in patients who were randomized to empagliflozin 10 mg vs placebo and standard of care therapy (HR, 0.79; 95% CI, 0.69-0.90; P < 0.0003). A total of 5988 patients were randomized with a median follow-up of 26 months. Standard of care therapy included 80% of patients receiving renin angiotensin inhibitor or ARNI, 38% receiving MRA, 86% receiving b- blocker, and 70% receiving statins in the placebo arm, which was not significantly different from the empagliflozin randomized group. The reduction in the primary composite endpoint was driven predominantly by a reduction in first hospitalization for HF (HR, 0.71; 95% CI, 0.60-0.83). The first hierarchical secondary endpoint of total (first and recurrent) HF hospitalization was significantly reduced (HR, 0.73; 95% CI, 0.61-0.88; P < 0.001) as was the second secondary endpoint, which was the slope of decline in glomerular filtration rate (-3.3 mL/min/1.73 m2 for those receiving empagliflozin vs -5.7 mL/min/1.73 m2 for those receiving placebo; P < 0.0001). This aligned with findings in the EMPEROR-Reduced trial. In contrast to Prospective Comparison of ARNI With ARB Global Outcomes in HF With Preserved Ejection Fraction (PARAGON-HF)[34] there was no heterogeneity for treatment effect for the primary endpoint relevant to sex or baseline LVEF on the basis of predefined tertiles of LVEF. There was also no heterogeneity for treatment benefit on the basis of the presence or absence of diabetes.[35] The safety profile was similar to that previously recognized in HFrEF patient cohorts. Additional data presented with an alpha protected pooled analysis of EMPEROR-Reduced and EMPEROR-Preserved suggest that empagliflozin is an agent that will be beneficial across a continuum of ejection fraction although benefit was not seen with ejection fraction > 65%.[36],[37]
The role of GLP-1RA and related agents in HFpEF might be clarified by ongoing studies.[30],[38],[39] On the basis of our meta-analysis (Table 1), use of SGLT2i is associated with a 29% reduction in hospitalization for HF. In contrast to the results in patients with HFrEF, the results in patients with HFpEF do not support a significant reduction in either all-cause or CV mortality or in reducing the composite kidney outcome.[7]
RECOMMENDATION
2. In adults with HF and LVEF > 40%, we recommend use of SGLT2i to reduce hospitalization for HF (Strong Recommendation; Moderate-Quality Evidence).
Practical tip
This recommendation is on the basis of the results of the EMPEROR-Preserved trial (empagliflozin 10 mg daily vs placebo in addition to recommended HF therapy) but trials using other SGLT2i are pending. The recommendation is intended for stabilized patients. SGLT2i are not indicated for the treatment of type 1 diabetes, or for patients receiving dialysis or with severely compromised renal function (eGFR < 20 mL/min/1.73 m2).
General discussion
High value is placed on use of therapies that reduce CV mortality and hospitalization for HF in well conducted randomized controlled trials. Medications such as ARNI and SGLT2i have clinical benefits in patients treated with ACEi or ARB, b-blockers, and MRA as background therapy. The mechanisms of action are complementary in patients with HFrEF and underscore a multidrug approach.
Preference is given to the use of pharmacotherapy in patients with symptomatic HFrEF regardless of New York Heart Association functional class. The writing group acknowledges lack of data that have directly compared dapagliflozin and empagliflozin in the management of HFrEF. Local accessibility to these agents and eGFR might provide guidance as to which agent is selected as a component of the 4 standard therapies for HFrEF. The writing group also acknowledges lack of evidence that has compared different strategies for the sequence in which guideline-directed medical therapies are prescribed.
Evidence from the recent Effect of Sotagliflozin on Cardiovascular Events in Patients With Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) trial [25] suggests that sotagliflozin (a sodium-glucose co-transporter 1/2 inhibitor, not yet available in Canada) could be used safely before discharge or shortly thereafter in patients with T2D who were hemodynamically stabilized after hospitalization for HF. Sotagliflozin significantly reduced the risk of achieving the primary endpoint of CV death, hospitalization for HF, or urgent visit for HF (51.0 vs 76.3 events per 100 patient-years; HR, 0.67; 95% CI, 0.52-0.85). The value of early initiation is a primary focus of 1 ongoing and 1 completed trial.[40],[41] The Empagliflozin 10 mg Compared to Placebo, Initiated in Patients Hospitalised for Acute Heart Failure (de Novo or Decompensated Chronic HF) Who Have Been Stabilised (EMPULSE) trial had not been published at the time of the final draft of this report and is not included in our meta-analysis.41 It included fewer than 600 patients, but using the win ratio approach (a new approach to the analysis of composite end points in clinical trials on the basis of the clinical priority attached to each component), it suggests that the use of SGLT2i in patients hospitalized for acute HF (HFrEF or HFpEF) can provide significant net clinical benefit, including reduced rates of rehospitalization and death within 90 days compared with placebo and regardless of the type of HF or diabetes status. In addition, improvements in quality of life metrics were seen and therapy was well tolerated compared with placebo with no cases of diabetic ketoacidosis (DKA) in either group. Although it would not have met our criteria for meta-analysis, another novel trial showed that patient-centred quality of life outcomes were improved with canagliflozin with similar results for patients with HFrEF and HFpEF, and for patients with and without T2D.[42]
References
5. O’Meara E, McDonald M, Chan M, et al. CCS/CHFS heart failure guidelines: clinical trial update on functional mitral regurgitation, SGLT2 inhibitors, ARNI in HFpEF, and tafamidis in amyloidosis. Can J Cardiol 2020;36:159-69.
7. Ali MU, Mancini GBJ, Fitzpatrick-Lewis D, et al. The effectiveness of sodium-glucose cotransporter 2 inhibitors and glucagon-like peptide-1 receptor agonists on cardiorenal outcomes: systematic review and meta-analysis. Can J Cardiol 2022;38:1201-10.
18. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995-2008.
21. Packer M, Anker SD, Butler J, et al. Empagliflozin in patients with heart failure, reduced ejection fraction, and volume overload: EMPEROR- Reduced Trial. J Am Coll Cardiol 2021;77:1381-92.
22. Anker SD, Butler J, Filippatos G, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med 2021;385:1451-61.
25. Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384: 117-28.
28 Martinez FA, Serenelli M, Nicolau JC, et al. Efficacy and safety of dapagliflozin in heart failure with reduced ejection fraction according to age: insights from DAPA-HF. Circulation 2020;141:100-11.
29. Jackson AM, Dewan P, Anand IS, et al. Dapagliflozin and diuretic use in patients with heart failure and reduced ejection fraction in DAPA-HF. Circulation 2020;142:1040-54.
30. KosiborodMN,JhundPS,DochertyKF,etal.Effectsofdapagliflozinon symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation 2020;141:90-9.
31. Serenelli M, Böhm M, Inzucchi SE, et al. Effect of dapagliflozin according to baseline systolic blood pressure in the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure trial (DAPA-HF). Eur Heart J 2020;41:3402-18.
32. Jhund PS, Solomon SD, Docherty KF, et al. Efficacy of dapagliflozin on renal function and outcomes in patients with heart failure with reduced ejection fraction: results of DAPA-HF. Circulation 2021;143:298-309.
33. Zannad F, Ferreira JP, Pocock SJ, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:819-29.
34. Solomon SD, McMurray JJV, Anand IS, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609-20.
35. Anker SD, Butler J, Filippatos G, et al. Effect of empagliflozin on cardiovascular and renal outcomes in patients with heart failure by baseline diabetes status: results from the EMPEROR-Reduced trial. Circulation 2021;143:337-49.
36. Packer M, Butler J, Zannad F, et al. Empagliflozin and major renal outcomes in heart failure. N Engl J Med 2021;385:1531-3.
37. Butler J, Packer M, Filippatos G, et al. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J 2022;43:416-26.
38. ClinicalTrials.gov. A study of tirzepatide (LY3298176) in participants with heart failure with preserved ejection fraction and obesity (SUM- MIT). Available at: https://clinicaltrials.gov/ct2/show/NCT04847557. Accessed November 29, 2021.
39. ClinicalTrials.gov. Research study to investigate how well semaglutide works in people living with heart failure and obesity (STEP-HFpEF). Available at: https://clinicaltrials.gov/ct2/show/NCT04788511. Accessed November 15, 2021.
40. ClinicalTrials.gov. Dapagliflozin and effect on cardiovascular events in acute heart failure – thrombolysis in myocardial infarction 68 (DAPA ACT HF-TIMI 68). Available at: https://clinicaltrials.gov/ct2/show/ NCT04363697. Accessed November 8, 2021.
41. Voors AA, Angermann CE, Teerlink JR, et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: a multi-national randomized trial. Nat Med 2022;28:568-74.
42. Spertus JA, Birmingham MC, Nassif M, et al. The SGLT2 inhibitor canagliflozin in heart failure: the CHIEF-HF remote, patient-centered randomized trial. Nat Med 2022;28:809-13.
