Introduction
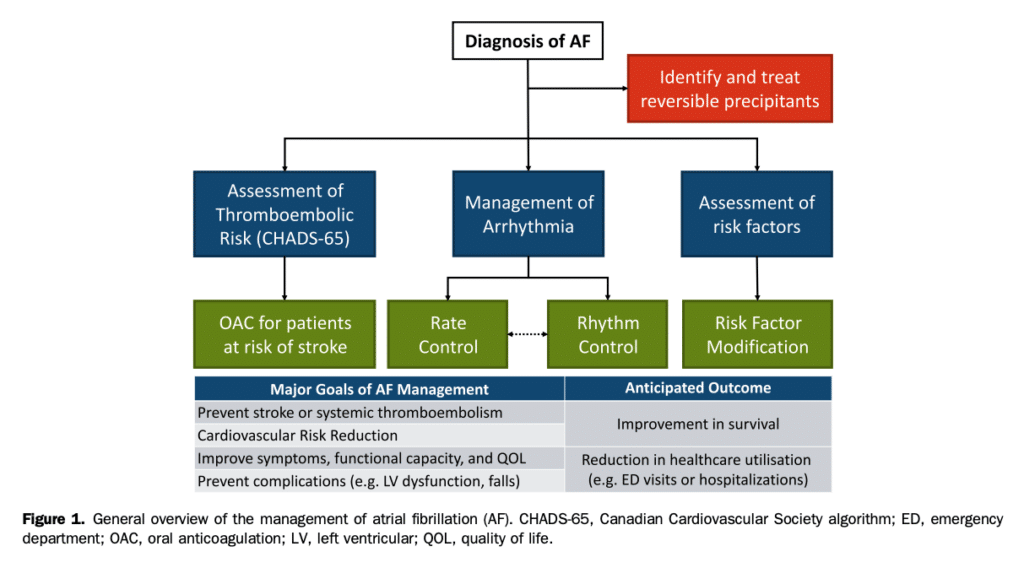
Atrial fibrillation (AF), the most common sustained cardiac arrhythmia, is associated with reduced quality of life (QOL), functional status, cardiac performance, and survival. The contemporary management of AF is centred on symptomatic improvement, diminution in morbidity and mortality (particularly the prevention of cardiomyopathy, and stroke/systemic embolism), and reduction in AF-related emergency department (ED) visits or hospitalizations (Fig. 1). The Canadian Cardiovascular Society (CCS) AF guidelines program was developed to aid clinicians in the management of these complex patients, as well as to provide direction to policy makers and health systems regarding the management of patients with AF. Beginning with the 1994 CCS consensus conference on AF, the CCS AF guidelines program has provided comprehensive guideline updates in 2004 and 2010, with focused updates on the basis of emerging evidence in 2012, 2014, 2016, and 2018.[1]–[8] The 2020 iteration of the CCS AF guidelines is a comprehensive renewal that integrates, updates, and replaces the past decade of guidelines, recommendations, and practical tips. It is intended to be used by practicing clinicians across all disciplines who care for patients with AF. The 2020 comprehensive AF guidelines address the following topics:
- Classification and Definitions
- Epidemiology
- Pathophysiology
- Clinical Evaluation
- Screening and Opportunistic AF Detection
- Detection and Management of Modifiable Risk Factors
- Integrated Approach to AF management
- Stroke Prevention
- Arrhythmia Management
- Sex Differences
- AF and Special Populations
Methodology
This document was developed in accordance with CCS best practices and the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach.[9] The primary panelists developed the scope of the document, identified topics for review, performed the literature review, evaluated the quality of the evidence, and drafted the recommendations. A systematic search was performed to identify relevant studies within each topic, including systematic reviews and metaanalyses. Draft recommendations were presented, reviewed, and refined by the primary panel. Final review was performed by the primary and secondary panels, with each recommendation achieving more than 90% agreement. Strength of recommendations and quality of evidence were evaluated according to the GRADE approach. Evidence derived from randomized clinical trials (RCTs) was initially estimated as high-quality evidence, with observational evidence initially deemed low-quality evidence. These estimates were further refined through detailed appraisal into 4 categorical grades (“high,” “moderate,” “low,” and “very low”) after consideration of the risk of bias, confounding, consistency of results, directness of evidence, precision, publication bias, magnitude of effect, and dose-response gradient. After this evaluation, the strength of recommendation was determined by considering the balance between desirable and undesirable effects (ie, risk-benefit), confidence in the magnitude of effect (quality of evidence), patient values and preferences, as well as resource considerations. The strength of recommendation was graded as “strong” (the desirable effects outweigh the undesirable effects, and therefore, most individuals will be best served by the recommended course of action) or “weak” (the desirable effects probably outweigh the undesirable effects, indicating that there is a need to consider the individual patient’s clinical details, circumstances, values, and preferences). Peer review of the guideline document was provided by external content experts, patient partners, and the CCS Guidelines Committee (outlined in the Acknowledgements section). The final draft was presented and approved by the CCS Executive Committee. For the constitution and roles of the primary and secondary panels, systematic review strategy, methods for formulating the recommendations, and evidence see the Supplementary Material and www.ccs.ca.
References
1. Canadian Cardiovascular Society Consensus Conference on Atrial Fibrillation. Can J Cardiol 1996;12(suppl A):1A-61A.
2. Andrade JG, Nattel S, Macle L. The Canadian Cardiovascular Society Atrial Fibrillation Guidelines Program: a look back over the last 10 years and a look forward. Can J Cardiol 2020;36:1839-42.
3. 2004 Canadian Cardiovascular Society Consensus Conference: Atrial Fibrillation. Can J Cardiol 2005;21(suppl B):9B-73B.
4. Cairns JA, Connolly S, McMurtry S, et al. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: prevention of stroke and systemic thromboembolism in atrial fibrillation and flutter. Can J Cardiol 2011;27:74-90.
5. Skanes AC, Healey JS, Cairns JA, et al. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol 2012;28:125-36.
6. Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol 2014;30:1114-30.
7. Macle L, Cairns J, Leblanc K, et al. 2016 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol 2016;32:1170-85.
8. Andrade JG, Verma A, Mitchell LB, et al. 2018 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol 2018;34:1371-92.
9. Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6.
