7. Integrated Approach to AF Management
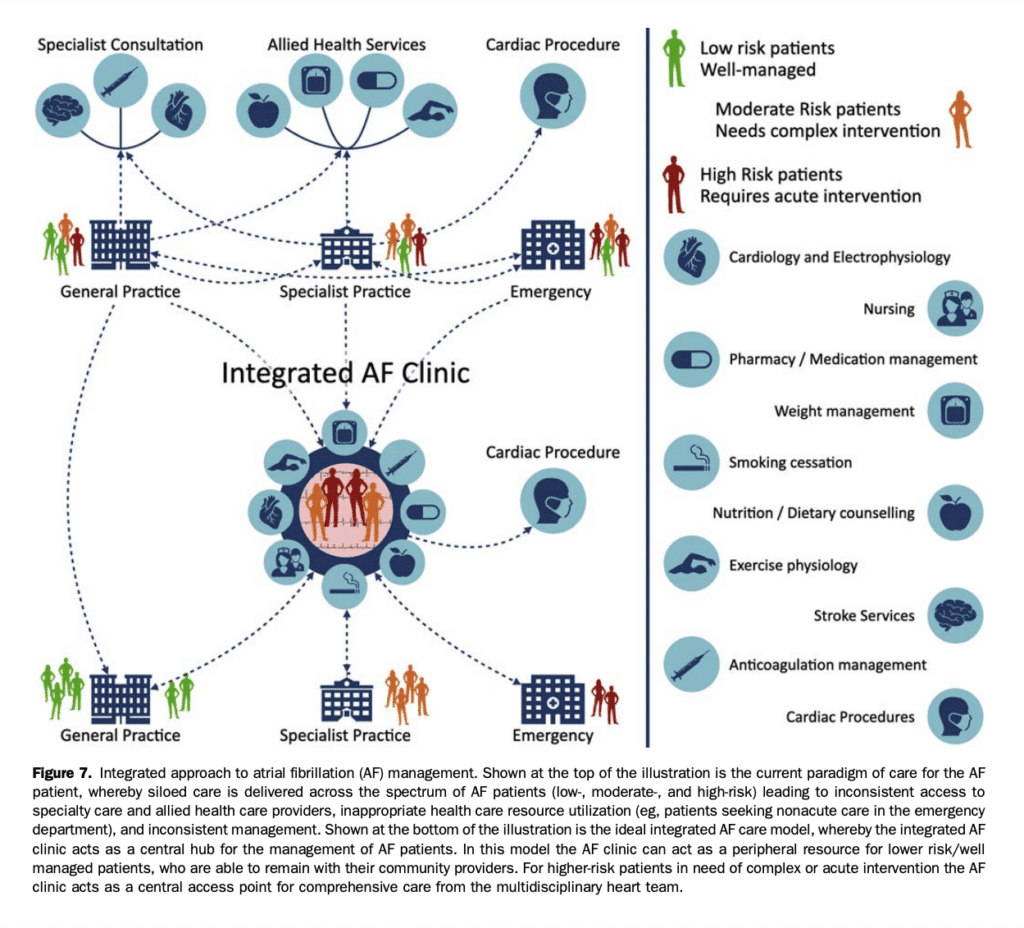
As with many other chronic cardiovascular conditions, the complex and multifaceted nature of AF necessitates a systematic approach to the management of the AF patient. Much of the initial management of AF can be provided by primary care providers with the support of specialist cardiology input to guide management decisions in selected AF patients who develop problems or complications during therapy. Dedicated multidisciplinary clinics specifically focused on integrated AF care have been developed to facilitate patient and provider education, provide advanced subspecialist treatment options, and deliver evidence-based care centred on chronic disease management principles. The systematic approach to patient care in integrated multidisciplinary AF clinic networks typically includes holistic protocol-driven management beyond the heart rhythm, with a particular focus on known care gaps in the domains of stroke prevention, transitions between rate and rhythm control, and coordination of heart rhythm procedures.[49],[133],[134] Although individually tailored to the needs of their communities,[135] multidisciplinary AF clinics are broadly on the basis of the principles of: (1) timely access to specialist care, to reduce adverse outcomes (eg, stroke or hospitalization) imposed by treatment delays; (2) knowledge translation, because improved understanding of AF facilitates active participation by the patient in their care pathway; (3) guideline adherence, in particular in the domains of stroke prevention and comorbidity management; and (4) integration with community care providers to enhance treatment cohesiveness and support care transitions (eg, from the ED to the specialty clinic and back to community care). A conceptual framework for the integrated approach to AF management is presented in Figure 7. Nonrandomized studies of specialized AF clinics have suggested that the greater coordination of care between specialist, nursing, and allied health interventions are associated with reduced wait times, enhanced transitions of care, improved adherence to guideline-based care, significant improvement in QOL, and more effective use of tertiary care resources.[136]–[139] These observational findings were supported by the results of a randomized single-centre study, which showed that a nurse-led multidisciplinary integrated care approach reduced cardiovascular death (HR, 0.28 vs usual care) and cardiovascular hospitalization (HR, 0.66 vs usual care), with resultant cost-effectiveness compared with standard care.[140],[141] Although these data were not replicated in a multicentre study of nurse-led vs usual care (HR, 0.85; 95% CI, 0.70-1.05; P ¼ 0.12 for the composite of cardiovascular death or cardiovascular hospitalization), a prespecified analysis showed significant benefit in experienced centres.[142]
Recommendation
12. We suggest a structured, integrated, multidisciplinary, patient-focused approach to care should be implemented for patients with AF (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation recognizes that AF is a multifactorial disease that requires long-term treatment. An integrated patient-focused teambased approach to care has been shown to improve guideline adherence, reduce adverse clinical outcomes such as hospitalization and mortality, and improve QOL.
7.1 Self-management, shared decision-making, and patient education
As with other chronic illness, self-management plays a crucial role in the longitudinal care of AF patients. Selfmanagement can be conceptualized as the “day to-day management of chronic conditions by individuals over the course of an illness” with an ultimate goal of improving health outcomes by enabling individuals to effectively manage their own illness.[143] Pragmatically, this involves shifting from the traditional provider-patient relationship to one in which the patient takes the responsibility for guiding their care in partnership with their health care providers (eg, shared decision-making).[144] Key areas of focus include medical management (eg, adherence to a therapeutic regimen), behaviour modification (eg, weight loss and exercise), and development of strategies to provide emotional and psychosocial support. Fundamental to the concept of self management is the patient’s perceived understanding about the cause(s), consequences, clinical manifestations, and controllability of their AF. Misalignment of the therapeutic interventions with the patient values and preferences can lead to dissatisfaction with therapy, nonadherence (not taking OAC as directed), and nonpersistence (therapy discontinuation), resulting in an increased risk of stroke.[145] Patient-centred care requires collaboration between clinicians and knowledgeable patients, considering the best available evidence in addition to the patient’s values and preferences.[145] Unfortunately, AF patients often have a poor understanding of the cause, consequences, and controllability of their AF, with particular deficiencies noted in the domain of stroke prevention.[145]–[147] Tailored patient education facilitates the construction of an accurate illness representation, improves patients’ illness-treatment coherence, corrects beliefs about therapeutic options and goals, improves treatment adherence, relieves disease-associated anxiety and stress, and promotes self-management.[146]–[148] However, the ideal educational strategy is uncertain. Despite written information, patient decision aids, and in-person education sessions all being commonly used, there is no consensus regarding the most effective structure, setting, or educator.[149]
Recommendation
13. We recommend that individualized goals of care and specific approaches to management should be developed in collaboration with patients and should consider their values and preferences to enhance engagement and improve adherence to long-term therapy (Strong Recommendation; Low-Quality Evidence).
14. We recommend that patients and care providers be supported with educational resources (in person and print/electronic) to enhance disease awareness and facilitate self-management (Strong Recommendation; Low-Quality Evidence).
7.2 Treatment adherence
Adherence has been defined as the “active, voluntary, and collaborative involvement of the patient in a mutually acceptable course of behaviour to produce a therapeutic result,” [150] which, in the case of pharmacotherapy can be further conceptualized as medication adherence (eg, the extent to which patients take their medications as prescribed) and persistence (eg, the duration of continuous use after index prescription). Unfortunately, nonadherence to pharmacotherapy is common for patients across the spectrum of cardiovascular diseases.[151],[152] In the AF population, adherence and persistence to stroke prevention therapies are suboptimal. Beyond the observation that approximately one-third of higher-risk AF patients fail to receive appropriate OAC,[153]–[155] of those who receive OAC approximately one-third of OAC-treated patients demonstrate suboptimal adherence.[156],[157] Furthermore, persistence in those who have started OAC treatment has been shown to be inadequate with 10% of those who have started VKA treatment failing to fill a second prescription, one-third discontinuing therapy within a year, and less than one-third continuing OAC after 5 years.[158] Likewise, in those who have started DOAC treatment the rates of discontinuation within a year have been reported to range between 13.6% and 52.7%.[159]–[161] Unfortunately, lower rates of OAC adherence has been associated with higher rates of all-cause mortality and stroke.[156],[157],[160]–[162] In consideration of the link between adherence and outcomes, it is paramount to pursue strategies that improve adherence and persistence. Although the focus is often limited to factors related to the patients themselves (eg, behavioural), nonadherence and nonpersistence are often multifactorial in origin. As such, any intervention that targets adherence and persistence must take into consideration factors related to the health care system (eg, lack of access or continuity of care), the patient-provider interaction (eg, a poor provider-patient relationship, poor communication, lack of patient education), the medical condition itself (eg, asymptomatic AF and the need for continued ongoing therapy), medical comorbidities (eg, physical disability, mental health disorders, or cognitive impairment), the therapeutic regimen (eg, complexity, medication side effects), or other socioeconomic factors (eg, low level of literacy, high medication cost, or lack of social support).[163] Solutions proposed to improve adherence and persistence are presented in Table 6. When used in isolation, these unimodal interventions have limited effect on adherence and clinical outcomes. Multimodal interventions, although complex, have shown the greatest benefit in improving outcomes. In the HF population an intensive pharmacist-led multimodal intervention increased medication adherence (78.8% vs 67.9%) and reduced ED visits/hospitalizations (relative risk [RR], 0.82; 95% CI, 0.73-0.93), saving USD$2960 in annual health care costs compared with usual care.[164] Likewise, a large cluster-randomized study showed that a multimodal intervention improved OAC prescription at 1 year compared with usual care (80% vs 67%).[165] Despite the absolute difference in OAC prescription being 9.1% (95% CI, 3.8-14.4) between groups, there was a > 50% reduction in stroke (HR, 0.48; 95% CI, 0.23-0.99; P ¼ 0.04). However, it is important to consider that the most effective interventions are often complex and time-consuming, which has limited their implementation outside of multidisciplinary clinical environments.
Recommendation
15. We recommend that adherence and persistence to pharmacotherapy be assessed at each clinical encounter and supported using patient-centred strategies (Strong Recommendation; Low-Quality Evidence).
Values and Preferences
This recommendation puts high value on the evidence that indicates poor long-term persistence and adherence with OAC treatment, as well as the recognition that strategies to improve persistence and adherence can substantially reduce the risk of stroke/ systemic embolism.
7.3 Electronic or mobile health
The term “eHealth” or “mHealth” commonly refers to the use of electronic media or mobile health technologies to provide or enhance the delivery of health care. eHealth can include the use of electronic interfaces (eg, Web sites or digital resources) along with mobile health devices (eg, handheld ECG devices or wearable smart devices).[166],[167] There are ample opportunities to incorporate eHealth throughout the provision of AF care (see section 5.1). Innovations in eHealth can improve access to services through outreach and telemedicine.[168] Smartphone applications and smart watches can measure the pulse using photoplethysmography or electrical sensors, providing an estimate of heart rate during daily activity and during arrhythmias. The future of AF care delivery will likely leverage a combination of point-of-care and other mHealth technologies to deliver a patient-centred experience.
References
49. Gladstone DJ, Bui E, Fang J, et al. Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke 2009;40:235-40.
133. Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med 2010;123:638-45.
134. Boodhoo L, Bordoli G, Mitchell AR, et al. The safety and effectiveness of a nurse led cardioversion service under sedation. Heart 2004;90:1443-6.
135. Cruz J, Mariano Z, Dorian P. Atrial fibrillation clinics in Canada: a nationwide project report. Can J Cardiol 2018;34:1219-24.
136. Gillis AM, Burland L, Arnburg B, et al. Treating the right patient at the right time: an innovative approach to the management of atrial fibrillation. Can J Cardiol 2008;24:195-8.
137. Elmouchi DA, VanOosterhout S, Muthusamy P, et al. Impact of an emergency department-initiated clinical protocol for the evaluation and treatment of atrial fibrillation. Crit Pathw Cardiol 2014;13:43-8.
138. Angaran P, Mariano Z, Dragan V, et al. The Atrial Fibrillation Therapies after ER visit: outpatient care for patients with acute AF – the AFTER3 study. J Atr Fibrillation 2015;7:1187.
139. Carter L, Gardner M, Magee K, et al. An integrated management approach to atrial fibrillation. J Am Heart Assoc 2016;5:e00295.
140. Hendriks JM, de WR, Crijns HJ, et al. Nurse-led care vs usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J 2012;33:2692-9.
141. Hendriks J, Tomini F, van Asselt T, Crijns H, Vrijhoef H. Cost effectiveness of a specialized atrial fibrillation clinic vs usual care in patients with atrial fibrillation. Europace 2013;15:1128-35.
142. Wijtvliet E, Tieleman RG, van Gelder IC, et al. Nurse-led vs usual-care for atrial fibrillation. Eur Heart J 2020;41:634-41.
143. Grady PA, Gough LL. Self-management: a comprehensive approach to management of chronic conditions. Am J Public Health 2014;104:e25-31.
144. Holman H, Lorig K. Patients as partners in managing chronic disease. Partnership is a prerequisite for effective and efficient health care. BMJ 2000;320:526-7.
145. Andrade JG, Krahn AD, Skanes AC, et al. Values and preferences of physicians and patients with nonvalvular atrial fibrillation who receive oral anticoagulation therapy for stroke prevention. Can J Cardiol 2016;32:747-53.
146. Lane DA, Ponsford J, Shelley A, Sirpal A, Lip GY. Patient knowledge and perceptions of atrial fibrillation and anticoagulant therapy: effects of an educational intervention programme. The West Birmingham Atrial Fibrillation Project. Int J Cardiol 2006;110:354-8.
147. Hernandez Madrid A, Potpara TS, Dagres N, et al. Differences in attitude, education, and knowledge about oral anticoagulation therapy among patients with atrial fibrillation in Europe: result of a selfassessment patient survey conducted by the European Heart Rhythm Association. Europace 2016;18:463-7.
148. Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the Necessity-Concerns Framework. J Psychosom Res 2008;64:41-6.
149. Wofford JL, Wells MD, Singh S. Best strategies for patient education about anticoagulation with warfarin: a systematic review. BMC Health Serv Res 2008;8:40.
150. Meichenbaum D, Turk DC. Facilitating Treatment Adherence: A Practitioner’s Guidebook. New York: Plenum Press, 1987.
151. Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J 2008;155:772-9.
152. Ruppar TM, Cooper PS, Mehr DR, Delgado JM, Dunbar-Jacob JM. Medication adherence interventions improve heart failure mortality and readmission rates: systematic review and meta-analysis of controlled trials. J Am Heart Assoc 2016;5:e002606.
153. Kakkar AK, Mueller I, Bassand JP, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS One 2013;8:e63479.
154. Camm AJ, Accetta G, Ambrosio G, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart 2017;103:307-14.
155. Huisman MV, Rothman KJ, Paquette M, et al. The changing landscape for stroke prevention in AF: findings from the GLORIA-AF registry phase 2. J Am Coll Cardiol 2017;69:777-85.
156. Shore S, Carey EP, Turakhia MP, et al. Adherence to dabigatran therapy and longitudinal patient outcomes: insights from the veterans health administration. Am Heart J 2014;167:810-7.
157. Alberts MJ, Peacock WF, Fields LE, et al. Association between onceand twice-daily direct oral anticoagulant adherence in nonvalvular atrial fibrillation patients and rates of ischemic stroke. Int J Cardiol 2016;215:11-3.
158. Gomes T, Mamdani MM, Holbrook AM, Paterson JM, Juurlink DN. Persistence with therapy among patients treated with warfarin for atrial fibrillation. Arch Intern Med 2012;172:1687-9.
159. Beyer-Westendorf J, Ehlken B, Evers T. Real-world persistence and adherence to oral anticoagulation for stroke risk reduction in patients with atrial fibrillation. Europace 2016;18:1150-7.
160. Lip GYH, Pan X, Kamble S, et al. Discontinuation risk comparison among ‘real-world’ newly anticoagulated atrial fibrillation patients: apixaban, warfarin, dabigatran, or rivaroxaban. PLoS One 2018;13: e0195950.
161. Ruigomez A, Vora P, Balabanova Y, et al. Discontinuation of nonvitamin K antagonist oral anticoagulants in patients with non-valvular atrial fibrillation: a population-based cohort study using primary care data from The Health Improvement Network in the UK. BMJ Open 2019;9:e031342.
162. Yao X, Abraham NS, Alexander GC, et al. Effect of adherence to oral anticoagulants on risk of stroke and major bleeding among patients with atrial fibrillation. J Am Heart Assoc 2016;5:e003074.
163. Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation 2009;119:3028-35.
164. Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med 2007;146:714-25.
165. Vinereanu D, Lopes RD, Bahit MC, et al. A multifaceted intervention to improve treatment with oral anticoagulants in atrial fibrillation (IMPACT-AF): an international, cluster-randomised trial. Lancet 2017;390:1737-46.
166. Pandya E, Bajorek BV. Assessment of Web-based education resources informing patients about stroke prevention in atrial fibrillation. J Clin Pharm Ther 2016;41:667-76.
167. Giebel GD, Gissel C. Accuracy of mHealth devices for atrial fibrillation screening: systematic review. JMIR Mhealth Uhealth 2019;7:e13641.
168. Linz D, Pluymaekers N, Hendriks JM. TeleCheck-AF for COVID-19. Eur Heart J 2020;41:1954-5.
