2. Management of PAD
2.1 Smoking cessation
PICO 2.1: Which smoking cessation interventions (behavioural and drug therapy) are efficacious at reducing MACE and MALE among patients with PAD?
Of all CV risk factors, tobacco exposure through cigarette smoking is the most strongly associated with the development and progression of PAD and its complications: MACE such as MI, stroke, CV death, and MALE. There is a solid foundation of data supporting a variety of smoking cessation interventions. Smoking cessation can prevent PAD and reduce MACE and MALE when PAD becomes symptomatic. Aside from behavioural counselling, pharmacological therapy should be considered, from nicotine replacement therapy (NRT) such as gum and patches, tobupropion and varenicline. Nicotine-containing e-cigarettes (EC) should also be given consideration. Behavioural therapy supporting pharmacotherapy does augment the 6-month quitting rate, but the relative risk (RR) is only 1.05-1.20, translating to a 20% quit rate compared with 17% when patients receive no support.[25] Behavioural therapy effectiveness is immensely variable in the literature and has been extensively reviewed in a recent Cochrane review of 312 RCTs with 250,563 participants. The RR of smoking cessation at 6 months is 1.44, ranging from 1.22 to 1.70 with a 6% background rate of quitting.[25] Drug therapies are few and the data are limited mainly to bupropion and varenicline. Bupropion shows an effect on quitting with a RR of 1.64 (additional 6 quitters per 100 after 6 months compared with a nonpharmacological approach).[26] This effect seems less than with varenicline (RR of 0.71).[26] Psychiatric adverse events seem more frequent with bupropion than with placebo (RR, 1.25).[26] A combination of bupropion with NRT or with varenicline marginally appears to enhance the quitting rate but the RRs are respectively 1.19 and 1.21, which are not significant.[27] In a network meta-analysis of 267 trials with 101,804 participants, varenicline and combination NRT (ie, combining 2 types of NRT such as patches, tablets, sprays, lozenges, and inhalers) vs placebo were most effective of all drug interventions (varenicline vs placebo odds ratio [OR], 2.88 [95% CI, 2.40 3.47]), accepting nausea as a frequent side effect. This translates to 1 extra quitter for 11 treated people.[28] Varenicline is similar to combination NRT (OR, 1.06 [95% CI, 0.75-1.48]), and superior to bupropion or single-use NRT, although each of these were superior to placebo (OR for bupropion vs placebo, 1.82 [95% CI, 1.60-2.06]) and NRT vs placebo (OR, 1.84 [95% CI, 1.71-1.99]). More recently, nicotine EC have been advocated to reduce smoking addiction. The most recent Cochrane review that compared nicotine EC with NRT showed a positive effect of nicotine EC on quitting with a RR of 1.53 (95% CI, 1.21-1.93) resulting in additional 3 quitters per 100 after 6 months. This effect was higher when compared with behavioural support only/no support, with a RR of 2.61 (95% CI, 1.44-4.74) resulting in additional 6 quitters per 100.[29] Among patients with PAD, a recent meta-analysis of 6 randomized trials involving 558 patients with PAD in which smoking interventions (behavioural counselling with or without NRT or a community intervention program promoting smoking reduction) were evaluated, suggested smoking cessation interventions increased the chance of quitting smoking (RR, 1.48 [95% CI, 0.84 2.61]),[30] although the wide CIs indicate a need for more RCTs. Furthermore, a high-quality trial of 124 patients with PAD included in this meta-analysis, in which intensive counselling with a minimum of 6 sessions was tested, was associated with a 2.97 (95% CI, 1.27-6.93) odds of smoking cessation, which was statistically significant.[31] Considering this, together with the positive effect of individual counselling, compared with usual care groups observed in a meta-analysis of smokers from the general population, which included 27 trials involving 11,100 people in which intensive counselling was effective at bringing about smoking cessation (RR, 1.57; 95% CI, 1.40-1.77), suggests this is an important and effective consideration for smoking cessation strategy for patients with PAD.[32]
Recommendations
- We recommend smoking cessation to prevent PAD, and to prevent MACE and MALE in patients with PAD (Strong Recommendation; Moderate-Quality Evidence).
- We recommend smoking cessation interventions ranging from intensive counselling, NRT, bupropion, varenicline, and sometimes nicotine EC (Strong Recommendation; High-Quality Evidence).
Values and Preferences
Smoking is one of the most potent risk factors for PAD and is associated with MACE and MALE complications. Studies were selected irrespective of whether the effect of smoking and smoking cessation were specifically focused on PAD patients but also CAD and cardiovascular disease patients or all of these regrouped subpopulations. High value was given to any intervention that led to a significant reduction or cessation of smoking, although the success rate of any given intervention was low compared with treatment of hypertension and dyslipidemia. The value of smoking cessation is considered high not only because of its effect on vascular disease, but also because of the profound effect on preventing many cancers and chronic obstructive pulmonary disease.
Practical Tip
Inquire at every clinical visit about the patient’s smoking status, even if abstinence has been achieved, and offer behavioural support, and present therapeutic options for smoking reduction, and how to access them to patients, because smoking is a major risk factor for CAD and PAD and smoking reduction decreases the risk of MACE and MALE.
2.2 Glucose control, diabetes, and PAD
2.2.1 Glucose control and PAD
PICO 2.2a: Does tight glycemic control (hemoglobin A1c < 7%) reduce incidence of MALE or need for revascularization in patients with PAD?
Patients with concurrent diabetes and PAD have a three- to fourfold increase in mortality and have a rate of amputation that is 5 times higher than patients with PAD without diabetes.[33]–[35] Although promising, no clear association between strict hemoglobin A1c control and reduction in MACE, MALE, or death in patients with PAD has been reported.
Recommendation
- We suggest that tight glycemic control might be beneficial for patients with PAD and diabetes in preventing MALE or need for revascularization (Weak Recommendation; Low-Quality Evidence).
2.2.2 Diabetes medications and PAD
PICO 2.2b: Do antihyperglycemic agents result in a reduction in MALE, revascularization, amputation, or MACE, in patients with PAD and diabetes?
The choice of antihyperglycemic agents in patients with PAD should be individualized to the patient’s wishes, preferences, and financial support/drug coverage. However, diabetes medication should be chosen to provide the optimal CV protection and reduction in MALE.[36] Unfortunately, very few hypoglycemic medications have been studied in patients with PAD, although some have shown more promise than others. Incretin-based selective inhibitors of dipeptidyl peptidase 4 (DPP-4) medications used in diabetes have not shown a reduction in MACE or MALE in PAD patients. However, a large observational trial of 82,169 patients who started receiving DPP-4 medication did show a 16% reduction in the development of PAD in patients with type 2 diabetes, and a subsequent 35% reduction in amputations among those with established PAD and type 2 diabetes.[37] This vascular protection was only shown in patients receiving combination therapy with metformin. A recent observational study reported a reduction in adverse limb outcomes with glucagon-like peptide 1 (GLP-1) agonists or DPP-4 inhibitors.[38] There is now a large body of evidence to support the use of the sodium-glucose cotransporter 2 (SGLT-2) inhibitors in patients with PAD and diabetes, in reducing mortality and MACE, and also MALE. In Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction (DECLARE-TIMI), the effects of dapagliflozin was examined in 17,160 patients with diabetes of whom 1025 had concurrent PAD. Patients with PAD and diabetes had higher baseline risk of MACE, hospitalization for heart failure, progression of renal disease, and MALE compared with patients without PAD. The benefit of dapagliflozin in reducing MACE, MALE, and death was consistent regardless of PAD diagnosis in this population. Furthermore, there was no increased risk of adverse limb outcomes in patients with PAD and diabetes randomized to dapagliflozin.[39] In the Canagliflozin Cardiovascular Assessment Study (CANVAS) trial, canagliflozin was associated with an increased risk of amputation among patients with diabetes and PAD, but this has not been observed with other SGLT-2 inhibitors such as empagliflozin and dapagliflozin. Two large meta-analyses reported no overall significant increased risk of amputation with SGLT-2 inhibitors as a class, with the only signal coming from canagliflozin but not the other agents (eg, empagliflozin, dapagliflozin).[40],[41]
Recommendation
- We recommend that patients with PAD and type 2 diabetes should be offered a SGLT-2 inhibitor compared with usual diabetic control because of the reduction in MACE without any risk of increased amputation (Strong Recommendation; High-Quality Evidence).
Recommendation
- We suggest that patients with PAD and diabetes might benefit from use of a GLP-1 agonist or DPP-4 inhibitor (Weak Recommendation; Low-Quality Evidence).
Practical Tip
Presently, there is no reason to suspect that empagliflozin or dapagliflozin increase the risk of PAD or lower limb amputations. The risk, or lack of risk, associated with canagliflozin remains to be established.
2.3 Lipid-lowering and PAD
PICO 2.3a: In patients with PAD, what is the role of cholesterol-lowering with statins, ezetimibe, niacin, or resins compared with placebo for the reduction of death, CV death, nonfatal MI, nonfatal stroke, or MALE?
PICO 2.3b: In patients with PAD, what is the role of PCSK-9 inhibitors compared with use of statins with or without ezetimibe for the reduction of death, CV death, nonfatal MI, nonfatal stroke, or MALE?
Patients with PAD constitute a very high-risk subset of patients with atherosclerotic vascular disease. There is strong and high-quality evidence supporting aggressive lipid-lowering with statins to reduce overall and CV mortality as well as major adverse CV and cerebrovascular events. There is also strong evidence to support this intervention for the purpose of avoiding MALE. There is moderate-quality evidence that aggressive lipid-lowering might improve patient outcomes such as pain-free walking time and overall ambulatory ability. See section 2.3 of the Supplementary Material for further evidence and explanation. PAD cohorts have been shown consistently to have high risk and high absolute event reductions with PCSK-9 inhibitors.[20] They benefit from significant event reductions, including adverse limb events, within 3 years, probably because of their far more substantial lipid-lowering compared with ezetimibe, and possibly because of additional benefit when lipoprotein(a) level is elevated. Patient outcomes such as pain-free walk time and overall ambulatory activity are not extensively studied but represent potential additional benefits that might promote acceptance and adherence to these lipid lowering therapies. Evidence for reduction of MALE or PAD patient-relevant outcomes is not yet available for icosapent ethyl. The additional use of this agent has been compared with placebo, not to additional use of ezetimibe or PCSK-9 inhibitors. Moreover, a decision to intensify lipid-lowering using the latter agents in patients receiving maximally tolerated statins might also affect triglycerides, thereby altering the criterion for consideration of icosapent ethyl, which is proven to reduce MACE in the absence of ezetimibe or PCSK-9 inhibitors. Accordingly, for the reduction of MACE, patient-physician decisions that accommodate patient preferences, priorities, and access issues will determine when it is appropriate to consider icosapent ethyl for the PAD patient. See section 2.3 of the Supplementary Material for further evidence and explanation.
Recommendation
- We recommend that patients with PAD qualify as statin-indicated patients and should receive lipid- modifying therapy for the reduction of death, CV death, nonfatal MI, nonfatal stroke (MACE), and MALE concordant with the recommendations in the 2021 Canadian Cardiovascular Society (CCS) guidelines for the management of dyslipidemia[42] (Strong Recommendation; High-Quality Evidence).
a. Maximally tolerated dose of statin therapy
b. Statin add-on therapies (ezetimibe and/or PCSK-9 inhibitors) if receiving maximally tolerated dose of statin therapy and the low-density lipoprotein cholesterol is 1.8 mmol/L, non-high-density lipoprotein cholesterol 2.4 mmol/L or apolipoprotein B100 0.7 mg/dL. - We recommend that patients with PAD, who, despite maximally tolerated dose of statin therapy have a tri-glyceride level of 1.5-5.6 mmol/L, should be considered for use of icosapent ethyl for the reduction CV death, nonfatal MI, and nonfatal stroke concordant with the recommendations in the 2021 CCS guidelines for the management of dyslipidemia[42] (Strong Recommendation; Moderate-Quality Evidence).
Values and Preferences
Statin add-on therapy and icosapent ethyl present numerous challenges with respect to cost and access for many patients. They also contribute to the burden of medications and complexity of therapy. Any decision to implement them should be made through open patient-physician discussion. Although not specifically validated in clinical trials, it is reasonable to approach the recommendations for statin add-on therapy in sequence with consideration of ezetimibe followed by PCSK-9 inhibitors if lipid thresholds are not met. But, as emphasized in the 2021 CCS guidelines for the management of dyslipidemia, patients whose levels are significantly above threshold in whom the typical reduction of low-density lipoprotein cholesterol by ezetimibe is not likely to achieve cholesterol levels below threshold might be served more effectively and efficiently by use of a PCSK-9 inhibitor as the add-on of choice while reserving ezetimibe if needed as a third, lipid-lowering agent.[42]
2.4 Hypertension
PICO 2.4a: How should hypertension be diagnosed in patients with lower extremity PAD?
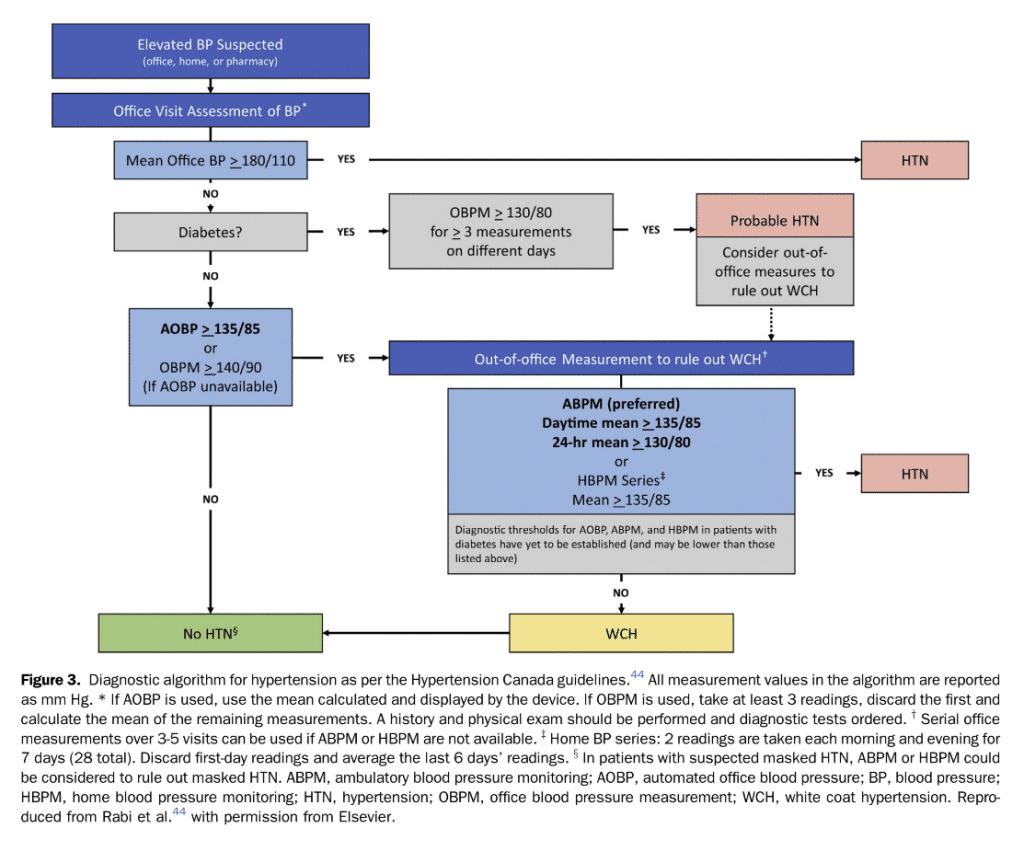
Because patients with PAD are at high risk of CV events, hypertension diagnosis and treatment is important as a risk reduction strategy. Most clinical studies that described the association between BP control and CV outcomes have used a 24-hour ambulatory BP monitoring (ABPM) assessment to establish a clinical diagnosis of hypertension.[43]–[45] Sequential home BP monitoring (HBPM) spaced throughout the day can be used as an alternative. The greater the number of recordings, the more accurately this reflects the true BP when averaged over multiple assessments.[46] Out-of-office BP measurements have a better prognostic value compared with office-based assessments.[43],[47] Moreover, 24-hour ABPM improves CV risk stratification compared with office-based BP assessments.[43],[48] Hence, the Hypertension Canada 2020 guidelines advocate for a standardized protocol in which BP is measured at 20 to 30 minute intervals throughout the day.[44] If ABPM monitoring is unavailable or not tolerated, HBPM can be used as an alternative. Hypertension can be diagnosed after multiple out-of-office assessments. Hypertension is diagnosed if the mean ambulatory daytime BP is 135/85 mm Hg, or if the 24-hour mean BP is 130/80 mm Hg (Fig. 3). Patients with PAD are at an elevated risk of future vascular events, and a target of 140/90 mm Hg should be considered as the treatment threshold.[49]
PICO 2.4b: In patients with PAD without an indication for a specific antihypertensive agent, what is the ideal BP target?
Antihypertensive therapy should be administered to patients with hypertension and PAD to reduce the risk of MI, stroke, heart failure, and CV death.[50],[51] There are no large-scale RCTs that specifically assessed BP targets in patients with lower extremity PAD. In a subgroup of patients with PAD from the Systolic Blood Pressure Intervention Trial (SPRINT),[52] intensive BP lowering to a systolic BP target < 120 mm Hg was associated with a reduction in the primary outcome of CV death and all-cause mortality. Because of the higher baseline risk among patients with PAD, the absolute risk reduction was larger in patients with PAD compared with those without PAD. However, intensive BP control also led to a greater absolute increased risk of adverse events in patients with PAD.
Recommendations
- We suggest favouring HBPM measurement or a 24-hour ABPM over office BP measurement for diagnosis and management of hypertension in patients with PAD. If there is a difference in BP measurements between arms, the higher value should be used for diagnosis and treatment considerations (Weak Recommendation; Low-Quality Evidence).
- We suggest that the approach to initiation and titration of antihypertensive agents should follow the Hypertension Canada guidelines[44] (Weak Recommendation; Low-Quality Evidence).
- We suggest treating hypertension to a target of less than 140/90 mm Hg in patients with PAD without compelling indications for specific agents or targets (Weak Recommendation; Low-Quality Evidence).
Practical Tip
In select patients, intensive systolic BP targets (< 120 mm Hg) might be considered. However, we suggest that caution be exercised if systolic BP is < 110 mm Hg because this is associated with an increase rate of adverse events (eg, MACE and MALE) in patients with PAD.
PICO 2.4c: In patients with PAD without an indication for a specific antihypertensive agent, what is the preferred approach to achieve optimal BP control?
Optimal hypertension management requires a holistic approach. Lifestyle modifications and pharmacological agents are the mainstay of treatment.
2.4.1 Lifestyle modification
Diet, exercise, weight management, alcohol reduction, stress management, and self-monitoring play an important role in managing BP. See Supplemental Table S1 for targets.
2.4.2 Pharmacological
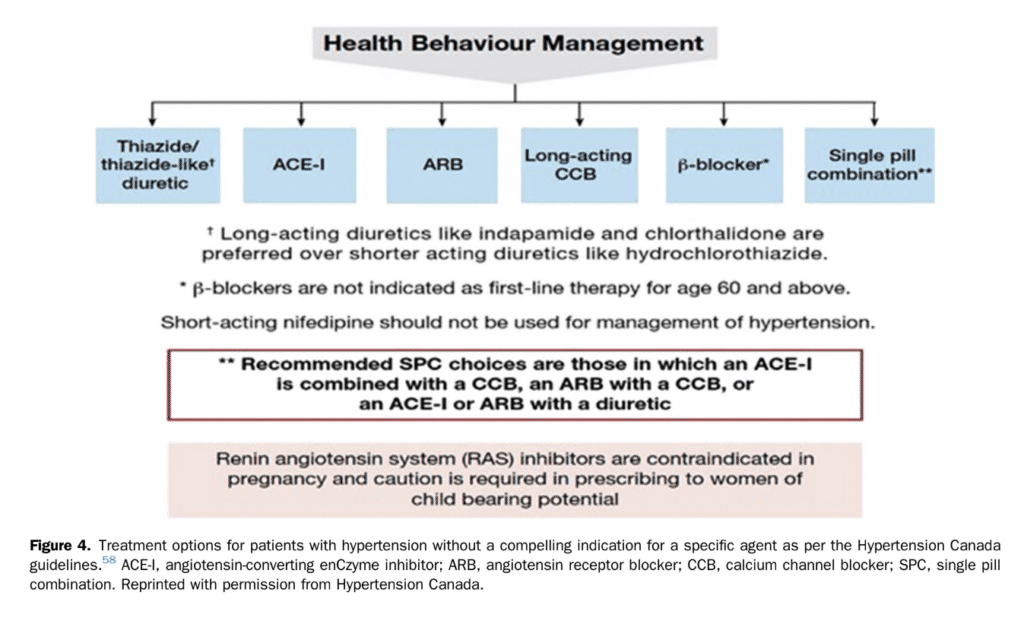
Angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), calcium antagonists, and diuretics are all suited for BP-lowering treatment in patients with PAD[53],[54]; see Figure 4, from Hypertension Canada. In the absence of contraindications, we recommend that patients with PAD and hypertension be treated with ACE inhibitors or ARBs as first choice agents. ACE inhibitors and ARBs have been shown to reduce MACE in patients with arterial peripheral vascular diseases.[55] ACE inhibitor or ARB use is also associated with reduced MACE among patients with critical limb ischemia.[56] Most patients with hypertension require multiple agents for optimal BP control. Use of combination pills therapy improves adherence, BP, and CV outcomes compared with usual pharmacological care.[55],[57]
Recommendation
- We recommend that PAD patients with hypertension be treated with ACE inhibitors or ARBs as the first choice in the absence of contraindications (Strong Recommendation; Moderate-Quality Evidence).
2.4.3 Special considerations in PAD patients
As mentioned previously, in the SPRINT trial, aggressive BP control among patients with PAD was associated with an increased risk of the primary outcome, CV death, and all-cause mortality compared with patients without PAD. As such, the optimal target is likely between 120 mm Hg and 140 mm Hg. A theoretical risk exists with the use of b-blockers in patients with limb ischemia. Previous guidelines have suggested avoiding the use of b-blockers in those with severe PAD. However, large systematic reviews on the topic have not shown increased harm with the use of b-blockers among patients with PAD. As such, they are not contraindicated and might be useful in PAD patients with concomitant CV disorders, where they are indicated as a second-line option.[59],[60]
2.5 Antithrombotic therapy
A substantial amount of evidence has emerged since the 2005 Canadian Cardiovascular Congress Consensus Conference for the management of PAD. The advent of newer thienopyridines and direct oral anticoagulants (DOACs), as well as their investigation within atherosclerotic PAD, has vastly expanded the tools available to practitioners. This has come with a concomitant shift in the understanding of the pathophysiology of lower extremity PAD. A large proportion of severe vascular occlusions are mediated by thrombotic occlusive disease, even in the absence of major atherosclerotic lesions, reframing PAD as a condition of “athero-thrombo-embolism” and informing the choice of antithrombotics investigated and used clinically. Lower extremity PAD is continually appreciated as but 1 manifestation of systemic atherosclerosis. As such, the efficacy of antithrombotics in lower extremity PAD are evaluated ac- cording to MACE and MALE outcomes. The benefit of antithrombotics in lower extremity PAD in conferring global vascular protection must be weighed against the risk of major and/or fatal bleeding.
2.5.1. Asymptomatic lower extremity PAD
PICO 2.5a: In adult patients with asymptomatic PAD does a single antiplatelet agent compared with placebo affect rates of MACE, MALE, or bleeding?
Patients with a low ABI, but without clinical limb symptoms or previous vascular intervention, are considered to have asymptomatic lower extremity PAD.
Recommendation
- We recommend against routine antithrombotic therapy (antiplatelet or anticoagulant) for patients with isolated asymptomatic lower extremity PAD (Strong Recommendation; High-Quality Evidence).
Practical Tips
- Atypical symptoms are common for lower extremity PAD, making a directed history (and consideration for noninvasive imaging when appropriate) essential for PAD classification.
- Patients with asymptomatic lower extremity PAD often have atherosclerotic coronary artery or cerebrovascular disease, and might merit antithrombotic therapy for these indications.
2.5.2 Stable symptomatic lower extremity PAD
PICO 2.5b: In adult patients with stable symptomatic lower extremity PAD (no recent or imminent revascularization), what is the optimal antithrombotic therapy considering the outcomes of MACE, MALE, or bleeding?
Patients with intermittent claudication, without recent (< 6 months) endovascular or surgical peripheral artery revascularization, and without acute symptoms of rest pain or tissue loss, are considered to have stable lower extremity PAD. Although single antiplatelet therapy has been the mainstay of antithrombotic therapy for symptomatic PAD patients,[61],[62] recent large trials that have tested low-dose DOACs together with aspirin have provided important new evidence.[63],[64]
Recommendations
- We recommend treatment with rivaroxaban 2.5 mg twice daily in combination with aspirin (80-100 mg daily) for management of patients with symptomatic lower extremity PAD who are at high risk for ischemic events (high-risk comorbidities such as polyvascular disease, diabetes, history of heart failure, or renal insufficiency) and/or high-risk limb presentation post peripheral revascularization, limb amputation, rest pain, ischemic ulcers) and at low bleeding risk (Strong Recommendation; High-Quality Evidence).
- We recommend combination treatment with rivaroxaban 2.5 mg twice daily and aspirin or single antiplatelet therapy for patients with symptomatic lower extremity PAD and low bleeding risk in the absence of high-risk limb presentation or high-risk comorbidities (Strong Recommendation; High-Quality Evidence).
Values and Preferences
This recommendation places high value on the overall Cardiovascular Outcomes for People Using Anticoagulation Strategies (COMPASS) trial findings, which showed a significant net clinical benefit with combination low-dose rivaroxaban and aspirin among a heterogenous patient population with PAD. Patients who place a high value on minimizing ischemic risk, such as MI, stroke, acute limb ischemia, or major vascular amputation, might opt for rivaroxaban 2.5 mg twice daily in combination with aspirin. Patients who place a high value on bleeding avoidance and minimizing pill burden might opt for single antiplatelet therapy alone.
Practical Tips
- Rivaroxaban 2.5 mg twice daily in combination with aspirin should be avoided in patients with strong cytochrome P450 family 3 subfamily A member 4 (CYP3A4) or p-glycoprotein medication interactions, those with recent stroke (< 1 month,) any previous hemorrhagic stroke, and with an estimated glomerular filtration rate < 15 mL/min. Although patients with severe heart failure (New York Heart Association classification III-IV or left ventricular ejection fraction < 30%) were excluded from the COMPASS trial, it might be reasonable to use rivaroxaban 2.5 mg twice daily in combination with aspirin, if otherwise indicated, and alternative etiologies for arterial occlusive disease have been excluded.
- At this time, the combination of rivaroxaban 2.5 mg twice daily and aspirin is not considered to be sufficiently equivalent to anticoagulation for management of atrial fibrillation or acute venous thrombosis. Optimal antithrombotic choices for these conditions can be found in the 2020 CCS/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation and the Thrombosis Canada clinical guides, respectively.[65],[66]
Recommendation
- We recommend single antiplatelet therapy with either aspirin (75-325 mg) or clopidogrel (75 mg) be considered for patients with symptomatic lower extremity PAD at high bleeding risk who remain eligible for antithrombotic therapy (Strong Recommendation; High-Quality Evidence).
Values and Preferences
This recommendation places a high value on the reduction of vascular events despite elevated bleeding risk. Patients at extremely high bleeding risk might not tolerate single antiplatelet therapy alone and might best be served by no antithrombotic therapy, particularly if vascular risk is low.
Recommendations
- We suggest that clopidogrel (75 mg daily)[61] should be the preferred agent when single antiplatelet therapy is deemed to be the optimal antithrombotic choice (Weak Recommendation; Moderate-Quality Evidence).
- We suggest that dual antiplatelet therapy (DAPT; aspirin and clopidogrel or aspirin and ticagrelor) be used for patients with symptomatic lower extremity PAD at high risk for vascular events, at low bleeding risk, and who have contraindications to rivaroxaban (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
This recommendation places greater weight on prevention of ischemic events (particularly coronary events) than on the risk of bleeding. The combination of ticagrelor and aspirin likely has greater ischemic benefit yet higher bleeding risk as contrasted with the combination of clopidogrel and aspirin, with choice of therapy directed by individual patient profile and preferences.
Practical Tips
Patients with recent (< 1 year) coronary revascularization and stable lower extremity PAD should have the choice of antithrombotic therapy guided by the 2018 CCS/Canadian Association of Interventional Cardiology focused update on the guidelines for the use of antiplatelet therapy,[67] although patients with symptomatic lower extremity PAD should merit particular consideration for ticagrelor and aspirin in combination.
Recommendation
- We recommend against the additional use of full-dose anticoagulation with antiplatelet therapy for the purpose of decreasing MACE and MALE events in patients with stable lower extremity PAD (Strong Recommendation; High-Quality Evidence).
Practical Tips
- Vascular and bleeding risk are not static and should be regularly reevaluated by primary care and vascular care practitioners, with dynamic adjustment of antithrombotic therapy as appropriate.
- Patients with stable lower extremity PAD who require full-dose anticoagulation for nonvascular in- dications might not require antiplatelet therapy. Although not robustly evaluated within the lower extremity PAD literature, analogous patients with stable CAD (no CV events in > 1 year) who required full dose anticoagulation experienced harm with the additional use of an antiplatelet agent with anticoagulation.[68]
2.5.3 Therapy after elective lower extremity revascularization
2.5.3.1 Endovascular revascularization
PICO 2.5c: In adult patients who undergo elective endovascular revascularization for lower extremity PAD, what is the optimal antithrombotic therapy to prevent MACE, MALE, bleeding, or need for repeat intervention, in the early postoperative period (within 12 months)?
The Antithrombotic Trialists’ Collaboration reported a numerical but not statistically significant reduction in MACE associated with single antiplatelet therapy vs placebo (odds reduction, 29%) after peripheral angioplasty.[69] A subsequent Cochrane review identified 2 small trials that compared aspirin and dipyridamole, respectively, vs placebo after endovascular revascularization, with pooled analysis showing a similar nonsignificant result for lesion patency at 6 months.[70] In the Management of Peripheral Arterial Interventions with Mono or Dual Antiplatelet Therapy (MIRROR) trial, DAPT using aspirin and clopidogrel was compared with aspirin alone in 80 patients after endovascular lower extremity revascularization. DAPT improved target lesion revascularization rates at 6 months (5% vs 8%; P 1⁄4 0.04) but not at 1 year.[25] Despite the lack of robust RCT data, DAPT after endovascular stenting is often extrapolated from the CV literature and mandated in trials investigating varying endovascular options.[71] Two small trials (n 1⁄4 160 and n 1⁄4 167, respectively) that compared full-dose oral anticoagulation with DAPT after endovascular revascularization showed no significant difference in lesion patency with increased bleeding events.[72],[73] Both trials were significantly underpowered with respect to their primary end point. Literature from patients who required full-dose anticoagulation and coronary artery stenting has shown full oral anticoagulant (OAC) in combination with single antiplatelet therapy can be an optimal strategy in select groups of patients.[74]–[77] Although less robust evidence exists after lower extremity stenting, the pilot Edoxaban in Peripheral Arterial Disease (ePAD) trial showed no significant difference in restenosis/reocclusion with edoxaban 60 mg compared with clopidogrel, largely on the background of aspirin therapy.[78] In the Vascular Outcomes Study of ASA [Acetylsalicylic Acid] Along with Rivaroxaban in Endovascular or Surgical Limb Revascularization for PAD (VOYAGER PAD) trial outlined in the following section, two-thirds of the patient population underwent endovascular intervention, making this the largest such trial conducted to date.[79]
2.5.3.2 Open revascularization
After infrainguinal arterial bypass surgery, a meta-analysis showed improved graft patency with aspirin, with or without dipyridamole (OR, 0.42; 95% CI, 0.22-0.83).[80] Data for alternative antiplatelet regimens are limited. In the Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral Arterial Disease (CASPAR) trial, clopidogrel and aspirin in combination vs aspirin alone was assessed in patients who underwent infrainguinal bypass surgery, and although no effect on MALE was seen in the overall population, a significant reduction was observed in a subgroup of patients who received prosthetic bypass grafts (hazard ratio [HR], 0.65; 95% CI, 0.45-0.95).[81] The 2 largest trials in open revascularization include the Dutch Bypass, Oral Anticoagulants or Aspirin (Dutch BOA) and VOYAGER PAD trial.[63],[82] Treatment with vitamin K antagonist (VKA) monotherapy (international normalized ratio, 3.0-4.5) compared with aspirin monotherapy did not significantly decrease graft occlusion or MACE, and significantly increased major bleeding (HR, 1.96; 95% CI, 1.42-2.71), in the overall Dutch BOA trial.[82] Subgroup analysis did, however, show improved patency with VKA in venous conduit bypass (HR, 0.69; 95% CI, 0.54-0.88), and with acetylsalicylic acid in nonvenous conduit grafts (HR, 1.26; 95% CI, 1.03 1.55).[82] Multiple smaller trials have assessed oral anticoagulation with single antiplatelet therapy vs single antiplatelet therapy or DAPT and have shown mixed results. The largest of these studies showed increased mortality with the combination of OAC and acetylsalicylic acid without improved graft patency.[83]–[85] The VOYAGER PAD trial randomized patients after endovascular or open revascularization to combination therapy with rivaroxaban 2.5 mg twice daily and aspirin or aspirin alone, with the option to additionally use clopidogrel up to a maximum of 6 months at the treating physicians’ discretion.[79] Compared with aspirin alone, the combination of rivaroxaban and aspirin reduced composite MACE and MALE events (HR, 0.85; 95% CI, 0.76-0.96), driven largely by a significant reduction in acute limb ischemia (HR, 0.67; 95% CI, 0.55-0.82).[79] Although there was no significant difference in the primary safety outcome of Thrombolysis in Myocardial Infarction (TIMI) major bleeding, the secondary safety outcome of the International Society on Thrombosis and Haemostasis major bleeding was increased (HR, 1.42; 95% CI, 1.10-1.84), albeit without significant increases in intracranial or fatal bleeding.[79] There was no significant heterogeneity in primary efficacy or bleeding outcomes on the basis of an open or endovascular approach.[79] Notably, most revascularization procedures were performed for the indication of worsening claudication (76.6%), and up to one-third of patients had critical limb ischemia.[79] Approximately 50% of the VOYAGER trial participants were given clopidogrel. The mean duration of clopidogrel use was 30 days, and the use of concomitant clopidogrel after revascularization did not alter the efficacy of rivaroxaban and aspirin compared with aspirin alone in reducing MACE or MALE events (P for interaction 1⁄4 0.92), nor rates of acute limb ischemia (P for interaction 1⁄4 0.93).[86] This was consistent for open and endovascular procedures. However, in those with longer courses of clopidogrel there was a trend toward increased major bleeding. More than 30 days of clopidogrel use came with a 2.71% absolute risk increase of major bleeding (HR, 3.20; 95% CI, 1.44-7.13), whereas < 30 days of clopidogrel conferred a 0.46% absolute risk increase of major bleeding (HR, 1.30; 95% CI, 0.68-2.47; P for interaction 1⁄4 0.07).[86]
Recommendations
- We recommend rivaroxaban 2.5 mg twice daily in combination with aspirin (80-100 mg daily), with or without short-term clopidogrel use, for patients with lower extremity PAD after elective endovascular revascularization (Strong Recommendation; Moderate-Quality Evidence).
- We recommend treatment with rivaroxaban 2.5 mg twice daily in combination with aspirin (80-100 mg daily) for patients with lower extremity PAD after elective open revascularization (Strong Recommendation; High-Quality Evidence).
Values and Preferences
This recommendation places high value on a single large well constructed RCT as opposed to multiple smaller low-quality studies. This recommendation also places high value on minimizing ischemic risk in the setting of acceptable increases to overall bleeding risk.
Practical Tips
- The additional use of clopidogrel (75 mg daily) with rivaroxaban 2.5 mg twice daily and aspirin (80-100 mg daily) can be considered in patients who undergo complex endovascular stenting. Should clopidogrel be used, it should be continued for a maximum of 30 days in the absence of other indications.
- Rivaroxaban 2.5 mg twice daily in combination with aspirin should be avoided in patients with strong CYP3A4 or p-glycoprotein medication interactions, stroke within 1 month, any previous hemorrhagic stroke, or estimated glomerular filtration rate < 15 mL/min.
- Patients who start treatment with rivaroxaban 2.5 mg twice daily in combination with aspirin after revascularization should preferably continue this therapy long-term in the absence of bleeding or ischemic manifestations, because previous revascularization represents a high-risk limb presentation for patients with stable lower extremity PAD.
Recommendation
- We suggest DAPT with aspirin (75-325 mg) and clopidogrel (75 mg) for at least 1 month in patients with lower extremity PAD after elective endovascular revascularization who are unable to receive low-dose rivaroxaban (Weak Recommendation; Very Low-Quality Evidence).
Values and Preferences
This recommendation places high value on expert opinion and indirect extrapolation from the CAD literature. Direct evaluation of DAPT after endovascular revascularization remains limited.
Practical Tip
Duration of DAPT might be affected by procedural factors such as use and complexity of stenting, as well as patient factors such as global ischemic and bleeding risk. It should be noted that the effect of stenting complexity or technology of drug-eluting stents (DES) on antithrombotic effect remains minimally studied.
Recommendation
- We suggest either a VKA or single antiplatelet therapy for patients with lower extremity PAD after elective open revascularization who are unable to receive low dose rivaroxaban (Weak Recommendation; Very Low-Quality Evidence).
Values and Preferences
This recommendation places greater value on overall trial results as opposed to discrepant results within patient subgroups. This recommendation is made acknowledging that robust evidence is not available to guide when VKA or single antiplatelet therapy would be of greater ischemic benefit, yet VKA therapy comes with a notably increased bleeding risk compared with single antiplatelet therapy. Therefore, this recommendation places a high value on expert opinion and intraoperative surgical decision-making for determining the optimal antithrombotic regimen in this scenario.
Practical Tips
- VKA might be particularly considered for patients who receive infrainguinal bypass using an autologous vein conduit with high-risk features including poor quality conduit, long conduit, disadvantaged distal runoff, or previous failed open revascularization.
- Full-dose DOAC therapy has not been studied in the setting of open peripheral revascularization. However, in high-risk patients deemed unsuitable for VKA therapy, full-dose DOAC may be considered as an alternative therapy.
2.5.4 Therapy after urgent/emergent lower extremity revascularization
PICO 2.5d: What is the optimal antithrombotic therapy among adult patients who undergo urgent or emergent revascularization (of any kind) for lower extremity PAD considering the outcomes of MACE, MALE, bleeding, or need for repeat intervention?
Limited comparative data exist to assess antithrombotic regimens in patients who require urgent or emergent revascularization despite their elevated risk of recurrent ischemic events and overall mortality.
Recommendation
- We suggest any of: (1) full-dose anticoagulation in combination with single antiplatelet therapy; (2) rivaroxaban 2.5 mg twice daily in combination with aspirin, with or without short-term use of clopidogrel; or (3) DAPT for patients with lower extremity PAD after urgent or emergent revascularization (Weak Recommendation; Very Low-Quality Evidence).
Values and Preferences
This recommendation acknowledges the lack of high-quality data informing antithrombotic treatment after urgent or emergent lower extremity revascularization and the noted heterogeneity in practice.[46]
Practical Tips
- Evaluation of the risk of re-thrombosis after urgent or emergent lower extremity revascularization should take into consideration the surgical procedure performed (ie, embolectomy or thrombectomy, bypass vs stenting), intraoperative findings (ie, residual distal occlusive disease, length and quality of conduit, infrapopliteal placement of conduit), as well as patient characteristics (ie, previous failed revascularizations, bleeding risk).[87] For patients deemed high-risk for re-thrombosis and low-risk for bleeding, full-dose anticoagulation in combination with single antiplatelet therapy should be particularly considered.
- In patients who require urgent or emergent lower extremity revascularization because of acute limb ischemia, care should be taken to rule out non-atherothromboembolic causes of limb ischemia, such as but not limited to cardioembolic cause, to inform the optimal antithrombotic therapy.
2.6 Exercise therapy for intermittent claudication
PICO 2.6: Among patients with PAD who have intermittent claudication, is supervised exercise, home, or community-based exercise therapy more efficacious than usual care, on outcomes of: total walking time and distance, claudication, need for revascularization, and quality of life?
Leg pain during activity is a major determinant of functional capacity among patients with PAD, negatively affecting their ability to perform activities of daily living and their quality of life. Thus, addressing functional impairment is crucial in the management of PAD patients with noncritical leg symptoms. A robust body of data support medical interventions aimed to improve walking and quality of life in patients with PAD. Because cilostazol is not yet available in Canada, and other potential medical interventions such as pentoxifylline, carnitine, propionyl-L carnitine, and chelation therapy have not been recommended in recent US[88] and European[89] guidelines because of lack of benefit or insufficient, potentially biased data, this section focuses on exercise therapy for the management of PAD patients with intermittent claudication.
Recommendations
- We recommend supervised exercise programs as first-line therapy for patients with PAD and intermittent claudication, with the objective of improving maximal and pain-free walking distance and time, as well as quality of life (Strong Recommendation; High-Quality Evidence).
- We recommend that a structured home-based or community exercise program can be offered to improve leg symptoms and quality of life when supervised exercise programs are not available, or not desired by the patient (Strong Recommendation; High-Quality Evidence).
- We recommend that walking should be the preferred form of exercise in exercise programs for intermittent claudication (Strong Recommendation; High-Quality Evidence).
Recommendations
- We suggest that, in patients with intermittent claudication who are unable to pursue walking exercise therapy, other forms of exercise such as cycle ergometer, arm ergometer, pole-striding, Nordic walking, or dynamic leg exercises can also be beneficial to improve leg symptoms (Weak Recommendation; Moderate-Quality Evidence).
- We suggest that resistance training can be used in addition to, but not substitute, walking therapy in exercise programs for patients with intermittent claudication (Weak Recommendation; Moderate-Quality Evidence).
Values and Preferences
High value is placed on a robust body of evidence that supports exercise therapy as first-line for improvement of walking, claudicant leg symptoms, and quality of life in patients with PAD. Preference is given to supervised exercise therapies that use walking as the primary form of exercise, but emerging evidence also supports home-based or community-based exercise as long as structure and guidance are provided throughout the duration of the program, as well as other forms of exercise when walking cannot be achieved or is not desirable.
Practical Tips
- Exercise habits should be asked at every visit with a health practitioner.
- Every patient with intermittent claudication should be referred to a supervised exercise rehabilitation program tailored to individuals with PAD, when available. When such a program is not locally available, structured guidance for home-based exercise should be provided by clinicians.
- Structured exercise guidance, either supervised or home or community-based, should include a minimum of 2 weekly sessions of at least 30 minutes duration, and be pursued for a minimum of 12 weeks of therapy.
- The authors acknowledge the lack of uniformity in exercise protocols for intermittent claudication. On the basis of our experience administering exercise therapy for PAD, patients are encouraged to walk on the treadmill or track for 8- to 9-minute bouts, with speed and/or incline sufficient to cause 3-4 of 5 claudicant pain toward the end of the 8 to 9 minute interval. Patients then rest until pain dissipates, and then resume a new bout of walking, repeating the cycles until 3 bouts are complete. Over the course of the program, walking speed and/or incline is adjusted by the therapist to continue achieving the aforementioned parameters.
- After patients complete the exercise program, they should be encouraged to continue walking for at least 30 minutes a day, at least 3 times a week, to maintain the walking and quality of life benefits gained during exercise therapy.
References
25. Hartmann-Boyce J, Livingstone-Banks J, Ordóñez-Mena JM, et al. Behavioural interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev 2021;1:CD013229.
26. Howes S, Hartmann-Boyce J, Livingstone-Banks J, Hong B, Lindson N. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2020;4:CD000031.
27. Brownrigg JRW, Hinchliffe RJ, Apelqvist J, et al. Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: a systematic review. Diabetes Metab Res Rev 2016;32(suppl 1):119-27.
28. Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2016;2016:CD006103.
29. Hartmann-Boyce J, McRobbie H, Lindson N, et al. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev 2021;4:CD010216.
30. Thanigaimani S, Drovandi A, Golledge J. A meta-analysis of randomised controlled trials evaluating the efficacy of smoking cessation interventions in people with peripheral artery disease. J Vasc Surg 2022;75:721-729.e7.
31. Hennrikus D, Joseph AM, Lando HA, et al. Effectiveness of a smoking cessation program for peripheral artery disease patients: a randomized controlled trial. J Am Coll Cardiol 2010;56:2105-12.
32. Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2017;3:CD001292.
33. Jude EB, Oyibo SO, Chalmers N, Boulton AJM. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care 2001;24:1433-7.
34. Beks PJ, Mackaay AJC, de Neeling JND, de Vries H, Bouter LM, Heine RJ. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn Study. Diabetologia 1995;38:86-96.
35. Resnick HE, Lindsay RS, McDermott MMG, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation 2004;109:733-9.
36. Stone JA, Houlden RL, Lin P, Udell JA, Verma S. Cardiovascular protection in people with diabetes. Can J Diabetes 2018;42(suppl 1): S162-9.
37. Chang CC, Chen YT, Hsu CY, et al. Dipeptidyl peptidase-4 inhibitors, peripheral arterial disease, and lower extremity amputation risk in diabetic patients. American Journal of Medicine 2017;130:348-55.
38. Lin DSH, Lee JK, Chen WJ. Major adverse cardiovascular and limb events in patients with diabetes treated with GLP-1 receptor agonists vs DPP-4 inhibitors. Diabetologia 2021;64:1949-62.
39. Bonaca MP, Wiviott SD, Zelniker TA, et al. Dapagliflozin and cardiac, kidney, and limb outcomes in patients with and without peripheral artery disease in DECLARE-TIMI 58. Circulation 2020;142:734-47.
40. Dicembrini I, Tomberli B, Nreu B, et al. Peripheral artery disease and amputations with sodium-glucose co-transporter-2 (SGLT-2) inhibitors: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 2019;153:138-44.
41. Huang CY, Lee JK. Sodium-glucose co-transporter-2 inhibitors and major adverse limb events: a trial-level meta-analysis including 51 713 individuals. Diabetes Obes Metab 2020;22:2348-55.
42. Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol 2021;37:1129-50.
43. Verdecchia P, Reboldi G, Porcellati C, et al. Risk of cardiovascular disease in relation to achieved office and ambulatory blood pressure control in treated hypertensive subjects. J Am Coll Cardiol 2002;39:878-85.
44. Rabi DM, McBrien KA, Sapir-Pichhadze R, et al. Hypertension Canada’s 2020 comprehensive guidelines for the prevention, diagnosis, risk assessment, and treatment of hypertension in adults and children. Can J Cardiol 2020;36:596-624.
45. Staessen JA, Thijs L, Fagard R, et al. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. JAMA 1999;282:539-46.
46. Agarwal R, Tu W. Minimally sufficient numbers of measurements for validation of 24-hour blood pressure monitoring in chronic kidney disease. Kidney Int 2018;94:1199-204.
47. Mulè G, Caimi G, Cottone S, et al. Value of home blood pressures as predictor of target organ damage in mild arterial hypertension. Eur J Cardiovasc Prev Rehab 2002;9:123-9.
48. Verdecchia P. Prognostic value of ambulatory blood pressure: current evidence and clinical implications. Hypertension 2000;35:844-51.
49. The SPRINT Research Group: A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015;373:2103-16.
50. Bavry AA, Anderson RD, Gong Y, et al. Outcomes among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the international VErapamil-SR/ trandolapril study. Hypertension 2010;55:48-53.
51. Feringa HHH, van Waning VH, Bax JJ, et al. Cardioprotective medication is associated with improved survival in patients with peripheral arterial disease. J Am Coll Cardiol 2006;47:1182-7.
52. Frary JMC, Pareek M, Byrne C, et al. Intensive blood pressure control appears to be effective and safe in patients with peripheral artery disease: the Systolic Blood Pressure Intervention Trial. Eur Heart J Cardiovasc Pharmacother 2021;7:e38-40.
53. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary. J Am Soc Hypertens 2018;12.579.e1-73.
54. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021-104.
55. Yusuf S, Joseph P, Dans A, et al. Polypill with or without aspirin in persons without cardiovascular disease. N Engl J Med 2021;384:216-28.
56. Armstrong EJ, Chen DC, Singh GD, Amsterdam EA, Laird JR. Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use is associated with reduced major adverse cardiovascular events among patients with critical limb ischemia. Vasc Med 2015;20:237-44.
57. Muñoz D, Uzoije P, Reynolds C, et al. Polypill for cardiovascular disease prevention in an underserved population. N Engl J Med 2019;381:1114-23.
58. Hypertension Canada. 2020 – 2022 Hypertension highlights: a practical guide informed by the Hypertension Canada Guidelines for the Prevention, Diagnosis, Risk Assessment, and Treatment of Hypertension. Available at: https://hypertension.ca/wp-content/uploads/2020/10/2020-22-HT-Guidelines-E-WEB_v3b.pdf. Accessed February 16, 2022.
59. Paravastu SCV, Mendonca DA, da Silva A. Beta blockers for peripheral arterial disease. Eur J Vasc Endovasc Surg 2009;38:66-70.
60. Soga Y, Iida O, Takahara M, Hirano K, Suzuki K, Kawasaki D. Beta-blocker treatment does not worsen critical limb ischemia in patients receiving endovascular therapy. J Atheroscler Thromb 2014;22:481-9.
61. CAPRIE Steering Committee: A randomised, blinded, trial of clopi-dogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet 1996;348:1329-39.
62. Antithrombotic Trialists’ (ATT) Collaboration, Baigent Colin, Blackwell Lisa, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849-60.
63. Hess CN, Debus ES, Nehler MR, et al. Reduction in acute limb ischemia with rivaroxaban versus placebo in peripheral artery disease after lower extremity revascularization: insights from VOYAGER PAD. Circulation 2021;144:1831-41.
64. Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018;391:219-29.
65. Andrade JG, Aguilar M, Atzema C, et al. The 2020 Canadian Cardiovascular Society/Canadian Heart Rhythm Society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol 2020;36:1847-948.
66. Thrombosis Canada. Clinical Guides. Available at: https://thrombosiscanada.ca/clinicalguides/#. Accessed September 12, 2021.
67. Andrade JG, Verma A, Mitchell LB, et al. 2018 Focused update of the Canadian Cardiovascular Society guidelines for the management of atrial fibrillation. Can J Cardiol 2018;34:1371-92.
68. Yasuda S, Kaikita K, Akao M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med 2019;381:1103-13.
69. Baigent C, Sudlow C, Collins R, Peto R. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 2002;324:71-86.
70. Robertson L, Ghouri MA, Kovacs F. Antiplatelet and anticoagulant drugs for prevention of restenosis/reocclusion following peripheral endovascular treatment. Cochrane Database Syst Rev Published online 2012;2012:CD002071.
71. Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX randomized trial. Circulation 2016;133:1472-83 [discussion: 1483].
72. Do DD, Mahler F. Low-dose aspirin combined with dipyridamole versus anticoagulants after femoropopliteal percutaneous transluminal angioplasty. Radiology 1994;193:567-71.
73. Pilger E, Lammer J, Bertuch H, et al. Nd:YAG laser with sapphire tip combined with balloon angioplasty in peripheral arterial occlusions: long-term results. Circulation 1991;83:141-7.
74. Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019;380:1509-24.
75. Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513-24.
76. Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423-34.
77. Mehta SR, Bainey KR, Cantor WJ, et al. 2018 Canadian Cardiovascular Society/Canadian Association of Interventional Cardiology focused update of the guidelines for the use of antiplatelet therapy. Can J Cardiol 2018;34:214-33.
78. Moll F, Baumgartner I, Jaff M, et al. Edoxaban plus aspirin vs dual antiplatelet therapy in endovascular treatment of patients with peripheral artery disease: results of the ePAD trial. J Endovasc Ther 2018;25:158-68.
79. Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med 2020;382:1994-2004.
80. Bedenis R, Lethaby A, Maxwell H, Acosta S, Prins MH. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database Syst Rev 2015;2015:CD00053.
81. Belch JJF, Dormandy J. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg 2010;52:825-33. 833.e1-2.
82. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral anticoagulants or Aspirin study): a randomised trial. Lancet 2000;355:346-51.
83. Sarac TP, Huber TS, Back MR, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg 1998;28:446-57.
84. Johnson WC, Williford WO. Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients peripheral arterial bypass procedures: a prospective randomized study. J Vasc Surg 2002;35:413-21.
85. Monaco M, di Tommaso L, Pinna GB, Lillo S, Schiavone V, Stassano P. Combination therapy with warfarin plus clopidogrel improves outcomes in femoropopliteal bypass surgery patients. J Vasc Surg 2012;56:96-105.
86. Hiatt WR, Bonaca MP, Patel MR, et al. Rivaroxaban and aspirin in peripheral artery disease lower extremity revascularization: impact of concomitant clopidogrel on efficacy and safety 2020;142:2219-30.
87. McClure GR, Kaplovitch E, Chan N, et al. A national Canadian survey of antithrombotic therapy after urgent and emergent limb revascularization. Can J Cardiol 2021;37:504-7.
88. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e686-725.
89. Aboyans V, Ricco JB, Bartelink MLEL, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39:763-816.
