3. Overview of the Management of Dyslipidemia in Primary Prevention
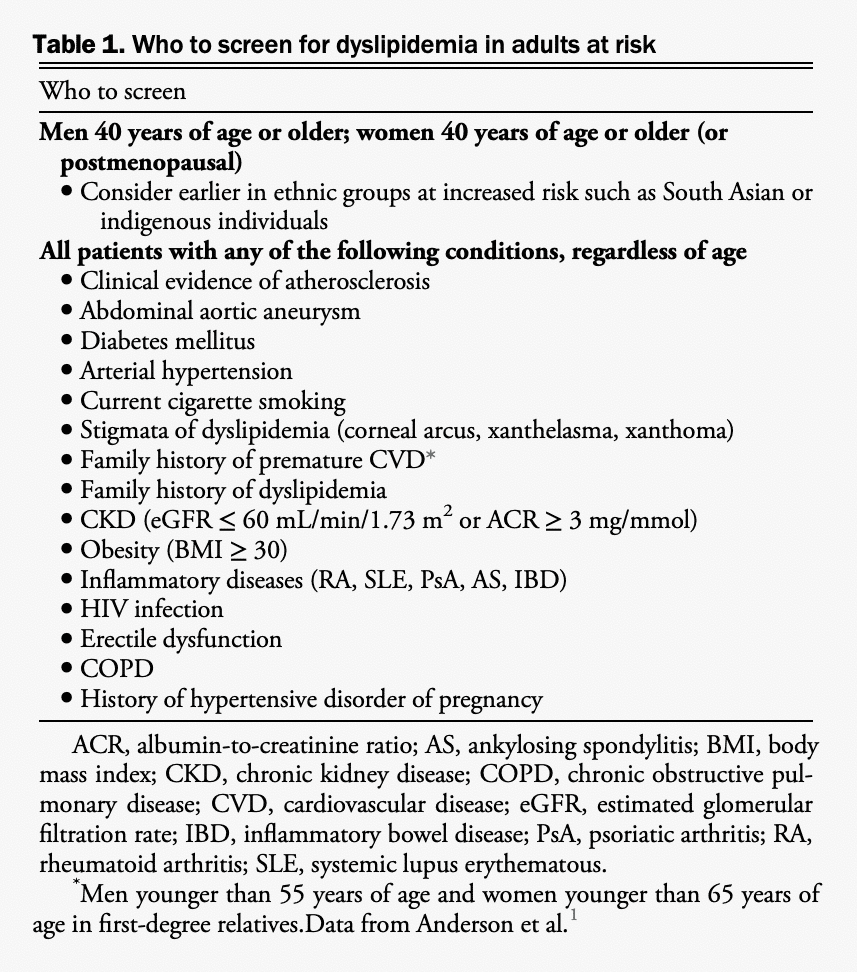
Screening
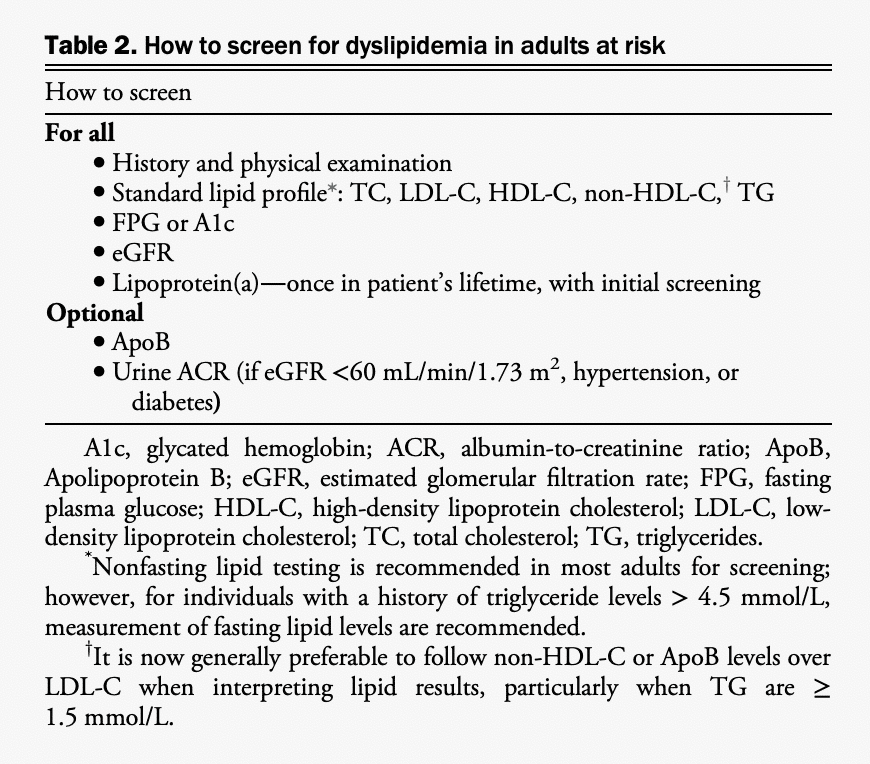
We determined that there was insufficient new evidence to recommend major changes to the approach of risk assessment in primary prevention. We continue to recommend lipid/lipoprotein screening (in either fasting or nonfasting state) for men and women older than 40 years of age or at any age with one of the specific conditions listed in Table 1. The nonfasting state is recommended (except for individuals with known triglycerides > 4.5 mmol/L) because it leads to minimal changes in relevant lipid levels and has no effect on apolipoprotein levels compared with the fasting state.[1] Table 2 provides a summary of the recommendations for how to screen patients. We maintain the recommendation for regular CV risk assessments using a validated risk model in Canada (either the Framingham Risk Score [FRS] or the Cardiovascular Life Expectancy Model [CLEM]) every 5 years for men and women aged 40-75 years to guide preventive care through shared decision-making with the patient. Among individuals 30-59 years of age without diabetes, the presence of a history of premature CV disease (CVD) in a first-degree relative (ie, 55 years or younger in male relatives and 65 years or younger in female relatives) increases an individual’s calculated FRS percent risk by approximately twofold.
Health behaviour interventions
Health behaviour modifications remain the cornerstone of chronic disease prevention, including CVD. Data from the INTERHEART study indicate that, in addition to the traditional risk factors (abnormal lipid levels, hypertension, smoking, and diabetes), abdominal obesity, dietary patterns, alcohol consumption, physical inactivity, and psychosocial factors are modifiable risk factors for MI worldwide in both sexes and at all ages.[2] Evidence from other large prospective cohort studies have also shown that combining low-risk health behaviours that include achieving and maintaining a healthy body weight, consuming a healthy diet, engaging in regular physical activity, smoking cessation, limiting alcohol consumption to no more than moderate, and ensuring a sufficient duration of sleep are associated with benefit for the primary prevention of CVD.[3],[4] We continue to recommend a Mediterranean dietary pattern, which has evidence of CV outcome benefit in systematic reviews and meta-analyses. Additionally, other dietary patterns that share important features such as the Portfolio dietary pattern,[5] Dietary Approaches to Stop Hypertension (DASH) dietary pattern,[6] low-glycemic index/glycemic load dietary pattern,[7] and plantbased dietary pattern,[8] as well as dietary patterns high in nuts,[9],[10] legumes,[10] olive oil,[9] fruits and vegetables,[11] total fibre,[12] and whole grains.[13] Dietary therapy using these means can be considered to augment drug therapy with statins; however, their benefits have been shown in terms of surrogate CV measures such as blood pressure and lipoproteins. We also continue to recommend that all adults should accumulate at least 150 minutes of moderate to vigorous aerobic activity per week. It might also be beneficial to add muscle- and bone-strengthening activities at least 2 days per week. Regular exercise has beneficial effects on diabetes risk, hypertension, and hypertriglyceridemia, and improves plasma levels of HDL-C.[14] A summary table of the expected CV outcomes and/or lipid benefits from various health behaviour changes is presented in Supplemental Appendix S4.
Health behaviour modifications remain the cornerstone of chronic disease prevention, including CVD. Data from the INTERHEART study indicate that, in addition to the traditional risk factors (abnormal lipid levels, hypertension, smoking, and diabetes), abdominal obesity, dietary patterns, alcohol consumption, physical inactivity, and psychosocial factors are modifiable risk factors for MI worldwide in both sexes and at all ages.[2] Evidence from other large prospective cohort studies have also shown that combining low-risk health behaviours that include achieving and maintaining a healthy body weight, consuming a healthy diet, engaging in regular physical activity, smoking cessation, limiting alcohol consumption to no more than moderate, and ensuring a sufficient duration of sleep are associated with benefit for the primary prevention of CVD.[3],[4] We continue to recommend a Mediterranean dietary pattern, which has evidence of CV outcome benefit in systematic reviews and meta-analyses. Additionally, other dietary patterns that share important features such as the Portfolio dietary pattern,[5] Dietary Approaches to Stop Hypertension (DASH) dietary pattern,[6] low-glycemic index/glycemic load dietary pattern,[7] and plantbased dietary pattern,[8] as well as dietary patterns high in nuts,[9],[10] legumes,[10] olive oil,[9] fruits and vegetables,[11] total fibre,[12] and whole grains.[13] Dietary therapy using these means can be considered to augment drug therapy with statins; however, their benefits have been shown in terms of surrogate CV measures such as blood pressure and lipoproteins. We also continue to recommend that all adults should accumulate at least 150 minutes of moderate to vigorous aerobic activity per week. It might also be beneficial to add muscle- and bone-strengthening activities at least 2 days per week. Regular exercise has beneficial effects on diabetes risk, hypertension, and hypertriglyceridemia, and improves plasma levels of HDL-C.[14] A summary table of the expected CV outcomes and/or lipid benefits from various health behaviour changes is presented in Supplemental Appendix S4.
Pharmacologic treatment
Studies consistently show a 20%-22% relative risk reduction for each 1 mmol/L reduction in LDL-C.[15] The absolute risk reduction is thus dependent on the baseline risk and the baseline LDL-C, because statin treatment will provide a greater absolute LDL-C lowering in those with higher baseline values. Therefore, we continue to recommend initiation of statin therapy for: (1) all high-risk patients (≥ 20% 10-year risk); or (2) intermediate-risk patients (10%-19.9%) when LDL-C is ≥ 3.5 mmol/L (or ApoB ≥ 1.05 g/L or non-HDL-C ≥ 4.2 mmol/L). In addition, among intermediate-risk individuals with several additional risk factors as evaluated in Heart Outcomes Prevention Evaluation (HOPE) 3[16] (men 50 years of age or older or women 60 years of age or older with 1 additional risk factor including low HDLC, impaired fasting glucose, increased waist circumference, cigarette smoking, hypertension) the evidence remains in favour of statin initiation to reduce the risk of CV events. The presence of other risk modifiers in intermediate-risk individuals also favours the use of statins (eg, high-sensitivity C-reactive protein ≥ 2.0 mmol/L, family history of premature coronary artery disease, high lipoprotein(a) [Lp(a)] ≥ 50 mg/dL [≥100 nmol/L] or coronary artery calcium score [CAC] > 0 Agatston units [AU]). For most low-risk subjects (FRS < 10%), health behaviour modification without pharmacotherapy is still recommended; however, the exceptions would be: (1) low-risk individuals with an LDL-C ≥ 5.0 mmol/L (or ApoB ≥ 1.45 g/L or non-HDL-C ≥ 5.8 mmol/L) who have a statin-indicated condition (likely a genetic dyslipidemia such as FH); or (2) individuals with an FRS of 5%-9% with an LDL-C ≥ 3.5 mmol/L (or ApoB ≥ 1.05 g/L or non-HDL-C ≥ 4.2 mmol/L), especially with other CV risk modifiers (eg, family history of premature coronary artery disease, Lp(a) ≥ 50 mg/dL [or ≥ 100 nmol/L] or CAC > 0 AU) because the proportional benefit from statin therapy will be similar to that in other treatment groups. Treatment of this group would follow the intermediate-risk approach. The treatment approach recommended for primary prevention patients is outlined in Figure 1. Finally, evidence continues to show the benefits of maintaining low levels of atherogenic lipoproteins throughout life and at any age and any level of risk. Even among primary prevention individuals at low 10-year risk, the benefit of lipid-lowering can be substantial, especially when LDL-C ≥ 3.5 mmol/L.[17] In addition, accumulating evidence suggests continued benefits of lipid-lowering for primary prevention in older adults (older than 75 years).[18]
Other statin-indicated conditions
We continue to recommend statin initiation for the following high-risk conditions (ie, “statin-indicated” conditions, even in the absence of a previous CV event: (1) CKD (except for patients receiving chronic dialysis) defined as patients with an estimated glomerular filtration rate < 60 mL/min/1.73 m2 and those with preserved estimated glomerular filtration rate in whom CKD is on the basis of an increased urinary albumin to creatinine ratio (≥ 3 mg/mmol) for at least 3 months’ duration; (2) diabetes mellitus in patients 40 years of age or older or 30 years of age or older with 15 or more years’ duration of diabetes, or the presence of microvascular complications; (3) abdominal aortic aneurysm > 3.0 cm or previous aortic aneurysm surgery.[1] Established ASCVD is also a statin-indicated condition, which is discussed in more detail later in these guidelines. The treatment approach for patients with a statin-indicated condition is summarized in Figure 2. All of the recommendations from the previous dyslipidemia guidelines that remain unchanged are provided in Supplemental Appendix S5.
New areas of focus
The review of literature and evidence assessment identified several areas for new and/or updated recommendations for primary prevention, specifically in: (1) the preventive care of women with hypertensive disorders of pregnancy; (2) the importance of lipoprotein measurement including non-HDLC, ApoB, and Lp(a) in assessing CV risk; (3) the role of CAC as a clinical decision-making tool for determining the need to initiate statin treatment; (4) the CV benefit of IPE in patients with triglycerides ≥ 1.5-5.6 mmol/L and a previous CV event or with diabetes and additional risk factors; and (5) the lack of CV benefit of omega-3 fatty acids from dietary sources or other formulations/supplements.
References
- Anderson TJ, Gregoire J, Pearson GJ, et al. 2016 Canadian Cardiovascular Society Guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2016;32:1263–82.
- Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004;364:937–52.
- Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and life-style. N Engl J Med 2000;343:16–22.
- Chiuve SE, McCullough ML, Sacks FM, Rimm EB. Healthy life-style factors in the primary prevention of coronary heart disease among men: benefits among users and nonusers of lipid-lowering and antihypertensive medications. Circulation 2006;114:160–7.
- Jenkins DJ, Jones PJ, Lamarche B, et al. Effect of a dietary portfolio of cholesterol-lowering foods given at 2 levels of intensity of dietary advice on serum lipids in hyperlipidemia: a randomized controlled trial. JAMA 2011;306:831–9.
- Schwingshackl L, Hoffmann G. Diet quality as assessed by the healthy eating index, the alternate healthy eating index, the dietary approaches to stop hypertension score, and health outcomes: a systematic review and meta-analysis of cohort studies. J Acad Nutr Diet 2015;115. 780-800.e5.
- Mirrahimi A, de Souza RJ, Chiavaroli L, et al. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts. J Am Heart Assoc 2012;1:e000752.
- Kwok CS, Umar S, Myint PK, Mamas MA, Loke YK. Vegetarian diet, Seventh Day Adventists and risk of cardiovascular mortality: a systematic review and meta-analysis. Int J Cardiol 2014;176:680–6.
- Estruch R, Ros E, Salas-Salvado J, et al. Primary prevention of cardiovascular disease with Mediterranean diet. N Engl J Med 2013;368:1279–90.
- Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:278–88.
- Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014;349:g4490.
- Threapleton DE, Greenwood DC, Evans CE, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2013;347:f6879.
- Tang G, Wang D, Long J, Yang F, Si L. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Car-diol 2015;115:625–9.
- Kodama S, Tanaka S, Saito K, et al. Effect of aerobic exercise training on serum levels of high density lipoprotein cholesterol: a meta-analysis. Arch Intern Med 2007;167:999–1008.
- Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA 2016;312:1289–97.
- Yusuf S, Bosch J, Dagenais G, et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31.
- Thanassoulis G, Williams K, Altobelli KK, et al. Individualized statin benefit for determining statin eligibility in the primary prevention of cardiovascular disease. Circulation 2016;133:1574–81.
- Gencer B, Marston NA, Im K, et al. Efficacy and safety of lowering LDL cholesterol in older patients: a systematic review and meta-analysis of randomised controlled trials. Lancet 2020;396:1637–43.
